Embed presentation
Downloaded 50 times
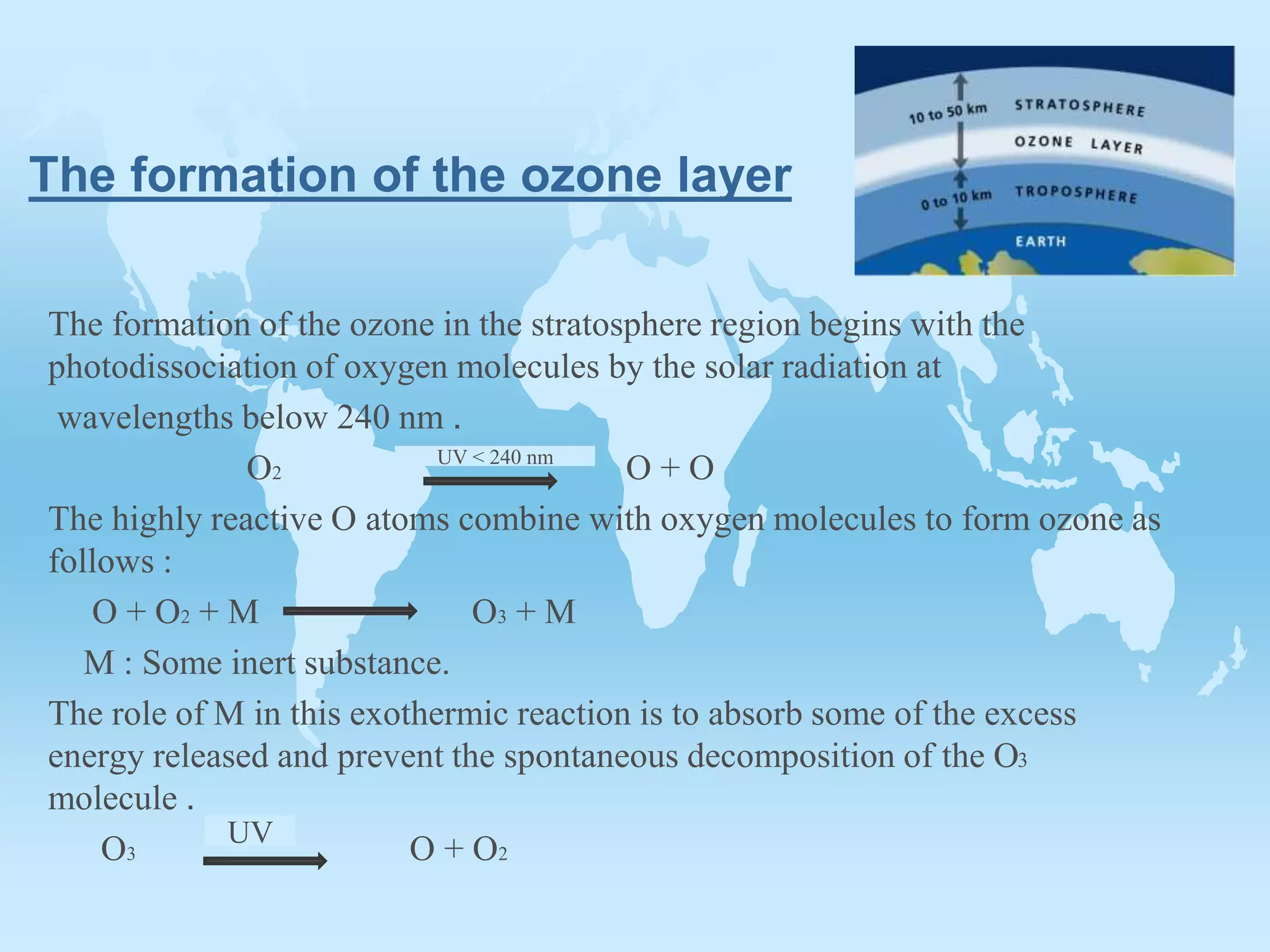

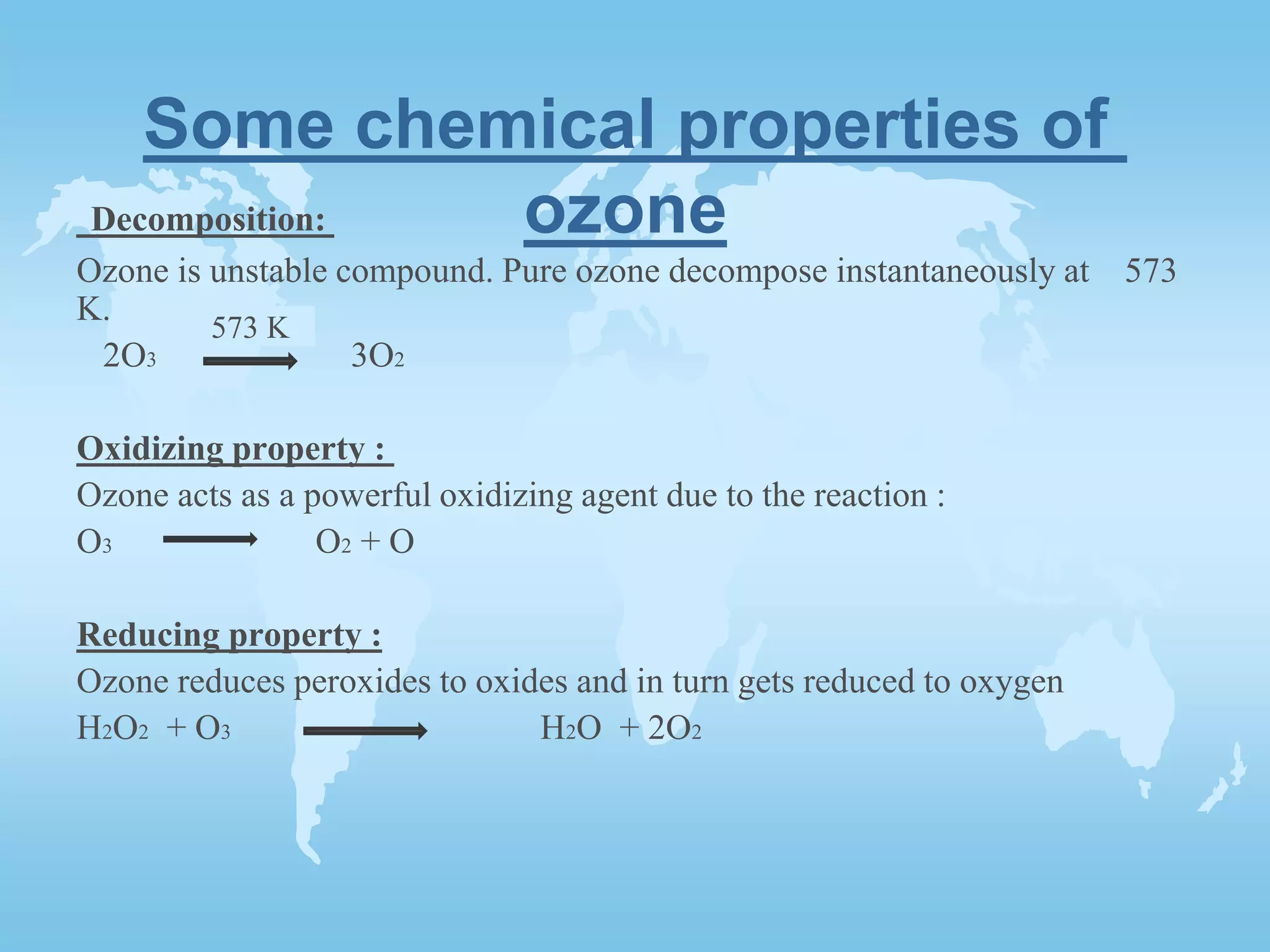
The ozone layer is formed in the stratosphere when solar radiation breaks oxygen molecules into oxygen atoms below 240nm wavelengths. The oxygen atoms then combine with oxygen molecules to form ozone molecules in an exothermic reaction where an inert substance absorbs excess energy to prevent ozone decomposition. Ozone is an unstable blue gas that decomposes into oxygen at high temperatures but acts as a powerful oxidizer and reducer in chemical reactions due to its unstable nature.


