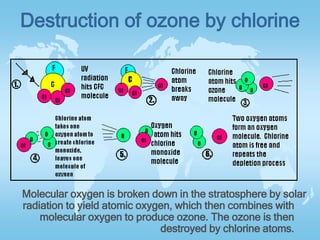
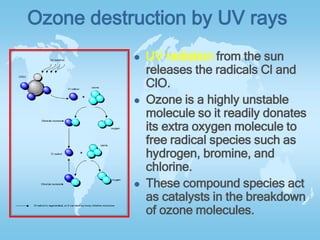
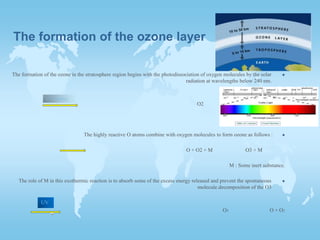
The two main factors negatively affecting the ozone layer are CFCs and NOx. CFCs released from refrigerants and air conditioners are destroyed by UV rays, releasing chlorine which then destroys ozone molecules. NOx both protects and destroys ozone depending on altitude - above 25km it destroys ozone, accounting for over 50% of total destruction, while below it protects ozone from being destroyed. The document also explains how ozone forms in the stratosphere through the combination of oxygen molecules and atoms produced by solar radiation breaking down oxygen.