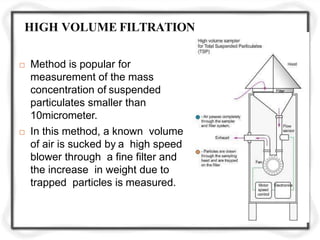
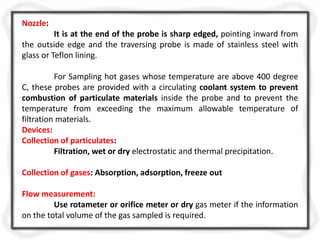
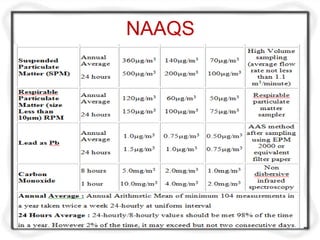
The document provides an extensive overview of air quality sampling and monitoring techniques, which include stack sampling, ambient air measurement, and various collection methods for gaseous and particulate pollutants. It outlines methods of sampling, analytical techniques, and emission norms for different types of vehicles, while also detailing site selection criteria and equipment used in the monitoring process. Additionally, it discusses ambient air quality standards and the Air Quality Index for assessing air quality in specific locations.