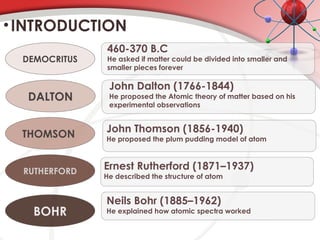
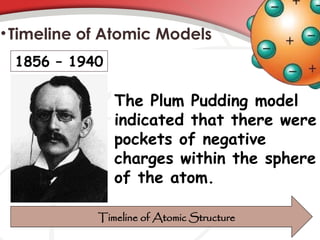
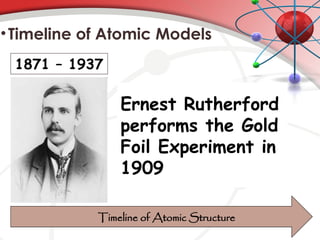
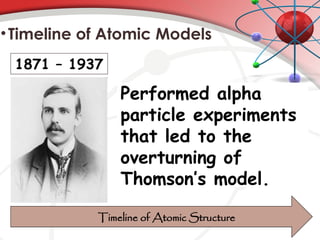
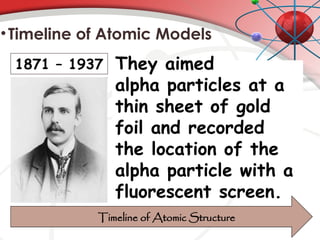
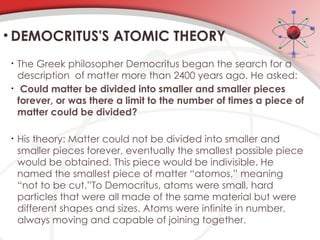
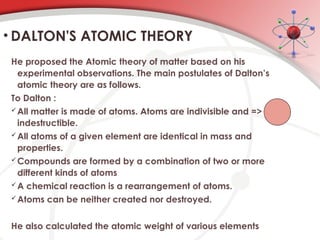
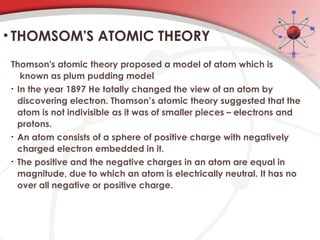
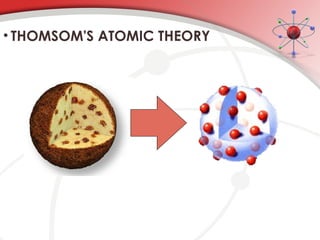
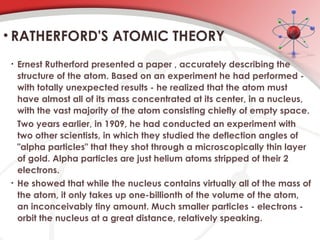
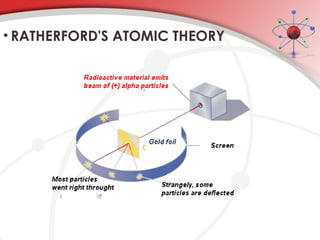
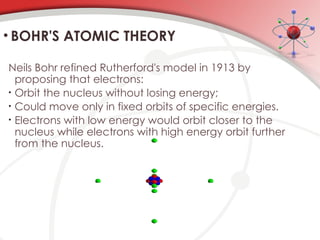
The document discusses the concept of matter, defining it as anything with mass and volume, and delves into the historical development of atomic theory from ancient philosophers like Democritus to modern scientists like Neils Bohr. Key figures, including John Dalton, J.J. Thomson, and Ernest Rutherford, contributed to the understanding of atomic structure, with significant models such as the plum pudding model and the nuclear model of the atom. The evolution of atomic theory illustrates how scientific understanding advances through observation, experimentation, and refinement.