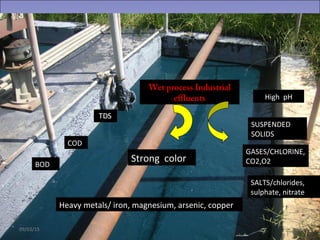
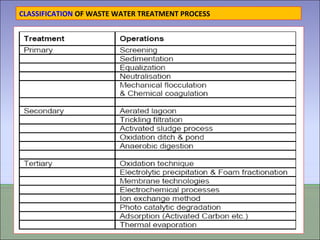
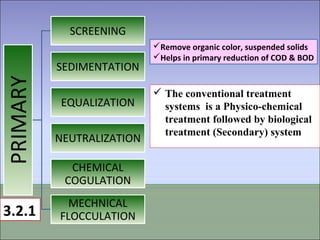
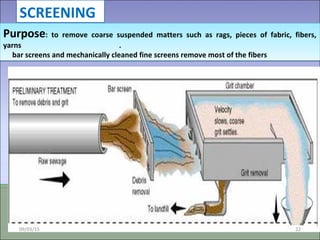
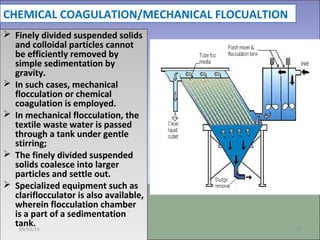
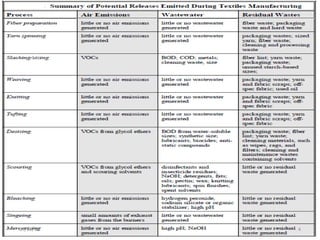
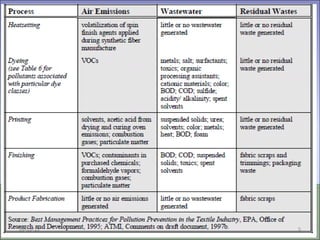
Two types of waste are generated from textile processing: process chemicals and fiber wastes. The nature of the waste depends on the type of textile facility, processes used, fibers, and chemicals. Textile effluent can include dispersible wastes mixed with other wastes in wastewater, hard-to-treat wastes that resist treatment and contain non-biodegradable or inorganic materials, and high-volume wastes like wash water, alkaline wastes, and warp sizes. Effluent can also contain hazardous or toxic wastes like metals, chlorinated solvents, and non-degradable surfactants. Common treatment processes include screening, sedimentation, equalization, neutralization, chemical



![Acute ToxicityAcute Toxicity
Single or multiple exposures in a short space of time (usually less than 24
hours).
Acute Toxicity of Textile Dyes
Skin Irritation
Vomit
Diarrhea
Reactive dyes can cause respiratory or skin sensitisation problems in
plant workers who manufacture the dyes and textile workers
Reactive Dyes + Human Serum Albumin [HSA]
Dye-HSA conjugate, which acts as an antigen. The antigen produces
specific immunoglobulin E (IgE) and, through the release of chemicals
such as histamine, causes allergic reactions
Single or multiple exposures in a short space of time (usually less than 24
hours).
Acute Toxicity of Textile Dyes
Skin Irritation
Vomit
Diarrhea
Reactive dyes can cause respiratory or skin sensitisation problems in
plant workers who manufacture the dyes and textile workers
Reactive Dyes + Human Serum Albumin [HSA]
Dye-HSA conjugate, which acts as an antigen. The antigen produces
specific immunoglobulin E (IgE) and, through the release of chemicals
such as histamine, causes allergic reactions
7
ToxicityToxicity](https://image.slidesharecdn.com/textileeffluenttreatment-150903123339-lva1-app6891/85/Textile-effluent-treatment-7-320.jpg)
![Chronic Toxicity
Non Genotoxicity
1.Water solubility
Water-soluble molecules are generally excreted rapidly by
a living organism
2.Water Insolubility
Due to the insolubility, toxicants gets large size particles
[0.1 to 3 mm] in the body which are not transported across
cell membranes.[e.g.] Pigments](https://image.slidesharecdn.com/textileeffluenttreatment-150903123339-lva1-app6891/85/Textile-effluent-treatment-8-320.jpg)
![Genotoxicity
Mutagens
Carcinogens
Teratogens
Toxicants reach the DNA (which resides in the nucleus of the
cell) in order for the chemical to interact with the DNA.
So the toxicants will be able to transport across the protective
cell membranes
Active species of most carcinogens, known as the ultimate
carcinogen, is an electrophile,
E.Nitrenium ion [R2N+]
Carbonium ion [R3C+]
Carcinogens attack a nucleophilic site in DNA, which may
be a carbon, nitrogen or oxygen atom, to form a covalent
chemical bond E + [DNA] or E–[DNA]
Genotoxicity
Mutagens
Carcinogens
Teratogens
Toxicants reach the DNA (which resides in the nucleus of the
cell) in order for the chemical to interact with the DNA.
So the toxicants will be able to transport across the protective
cell membranes
Active species of most carcinogens, known as the ultimate
carcinogen, is an electrophile,
E.Nitrenium ion [R2N+]
Carbonium ion [R3C+]
Carcinogens attack a nucleophilic site in DNA, which may
be a carbon, nitrogen or oxygen atom, to form a covalent
chemical bond E + [DNA] or E–[DNA] 9](https://image.slidesharecdn.com/textileeffluenttreatment-150903123339-lva1-app6891/85/Textile-effluent-treatment-9-320.jpg)