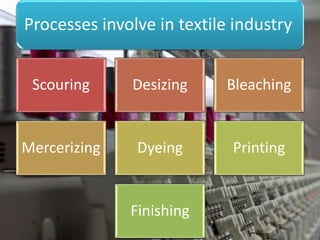
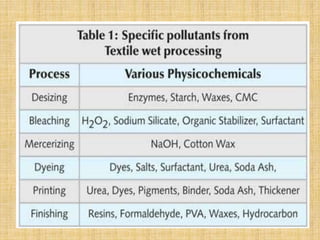
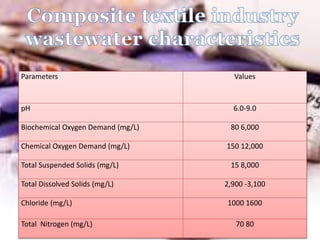
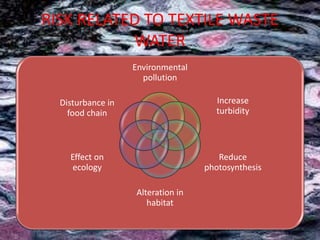
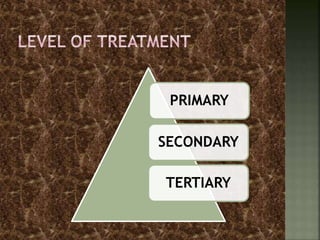
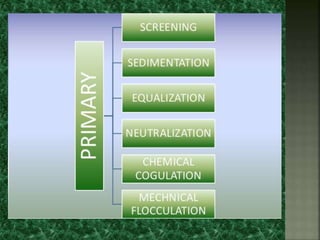
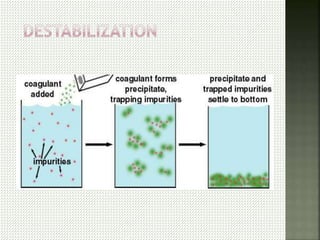
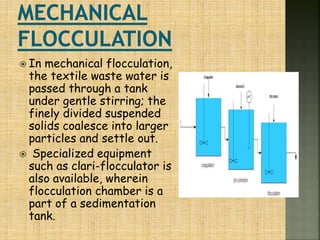
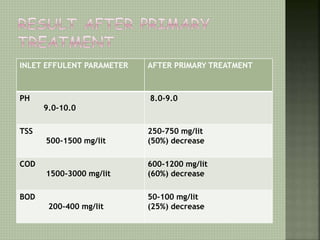
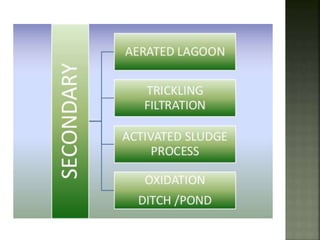
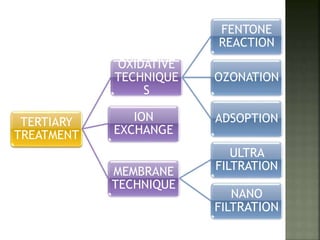
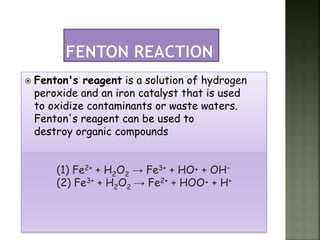
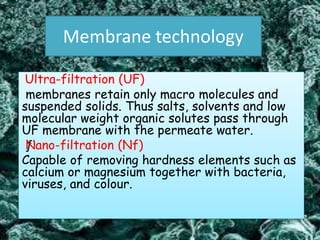
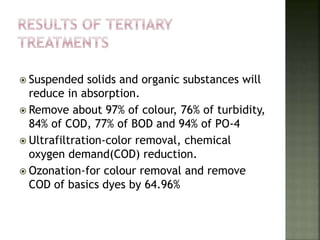
Textile wastewater treatment is essential due to the high levels of pollutants, including reactive dyes and chemicals, generated by textile industrial activities. Various treatment methods, such as primary, secondary, and tertiary processes, are employed to reduce biochemical oxygen demand (BOD), chemical oxygen demand (COD), and suspended solids. Advanced techniques like Fenton's reagent and membrane technologies are used to effectively remove toxic contaminants and improve water quality.