Embed presentation
Downloaded 19 times



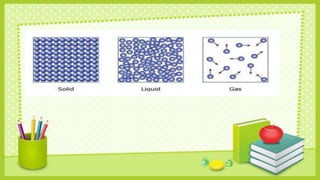







Matter can exist in three states - solid, liquid, and gas. The state depends on how closely packed the molecules are and how freely they can move. In solids, molecules are tightly packed and can only vibrate. In liquids, molecules have some space between them and move more freely. Gas molecules are most spaced out and move freely in any direction. Increases in temperature cause molecules to move faster and spread out more, potentially changing the state from solid to liquid to gas. Decreases in temperature have the opposite effect, causing molecules to slow down, pack closer together, and potentially change the state from gas to liquid to solid.











