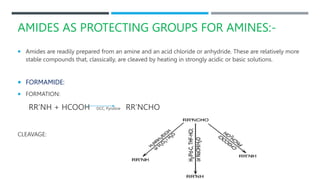
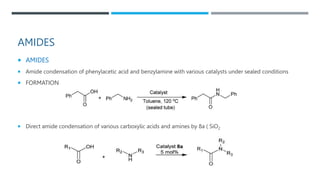
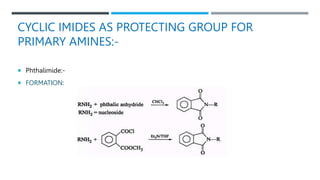
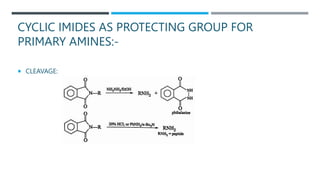
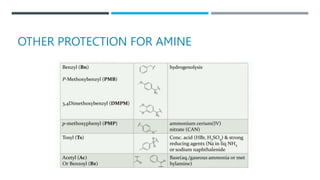
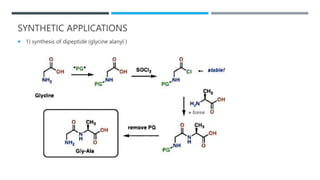
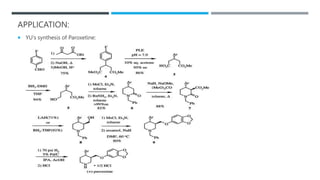
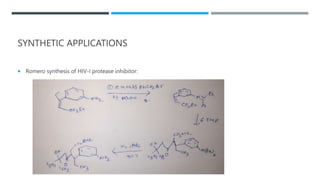
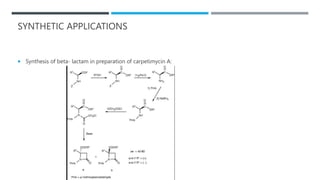
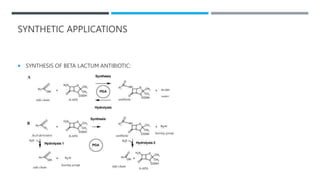
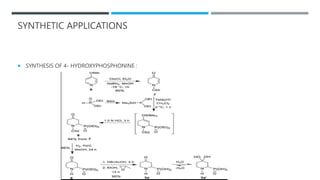
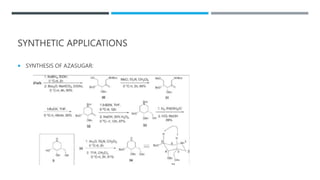
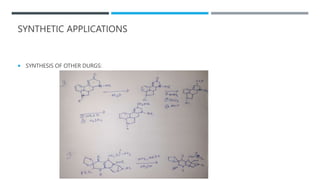
The document discusses various protecting groups used for amino groups in organic synthesis. It describes qualities of a good protecting group and highlights common protecting groups for amines like carbamates, amides, and cyclic imides. Specific protecting groups covered in detail include t-butyl carbamate, 9-fluorenylmethyloxycarbonyl, benzyloxycarbonyl, formamide, and phthalimide. The document concludes by providing examples of applications of protecting groups in peptide synthesis and synthesis of drugs.




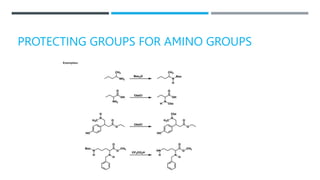
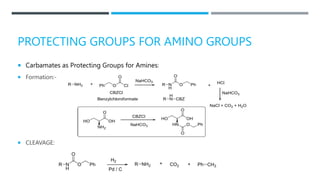

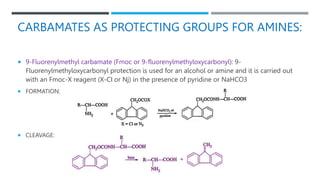
![BENZYL CARBAMATE [BENZYLOXY-CARBONYL OR
CBZ GROUP (RRNCOOCH2PH)]:-
FORMATION:
CLEAVAGES:](https://image.slidesharecdn.com/protectionfortheamine-230919083932-79713fdc/85/Protection-for-the-AMINE-pptx-12-320.jpg)