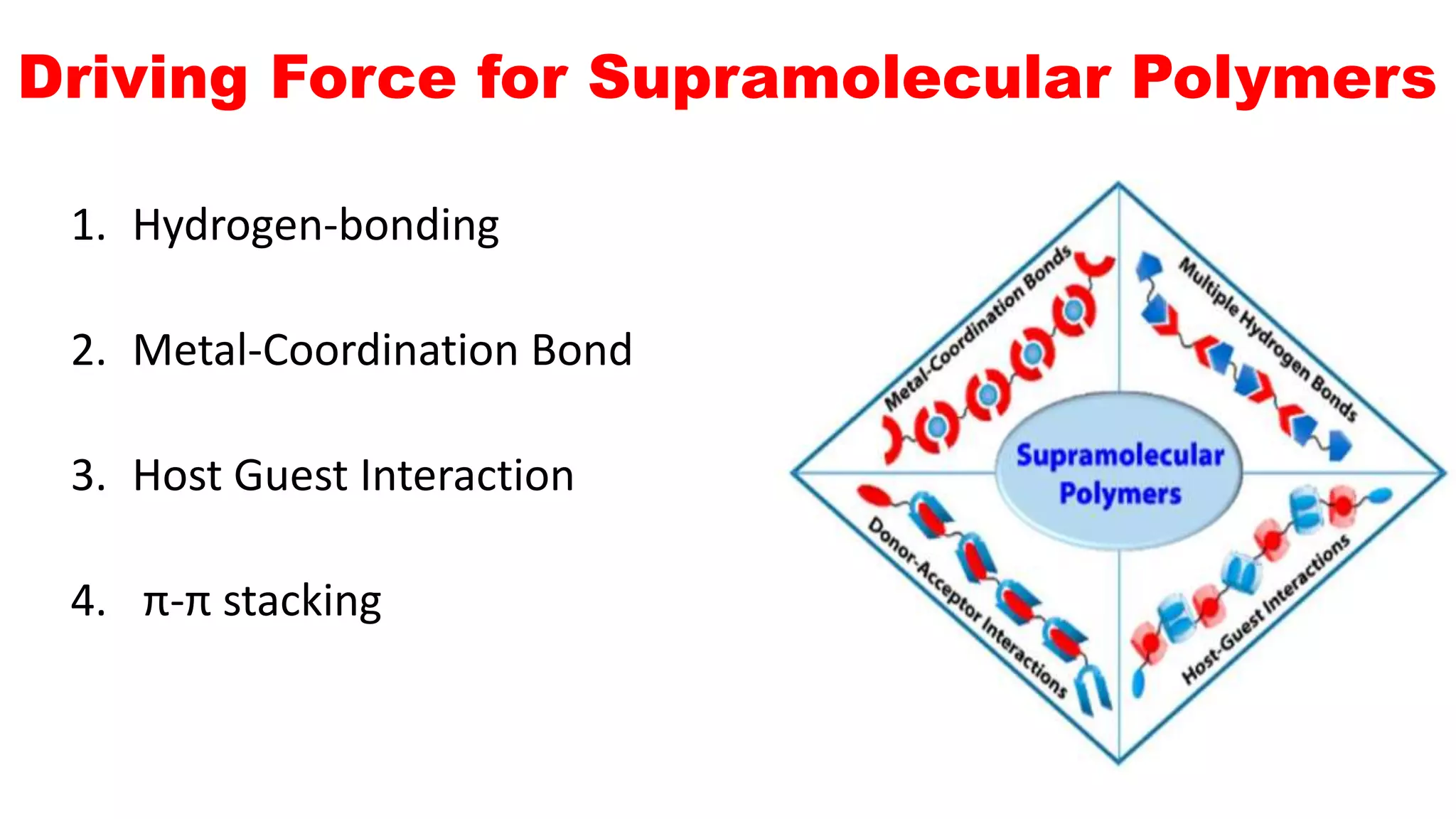
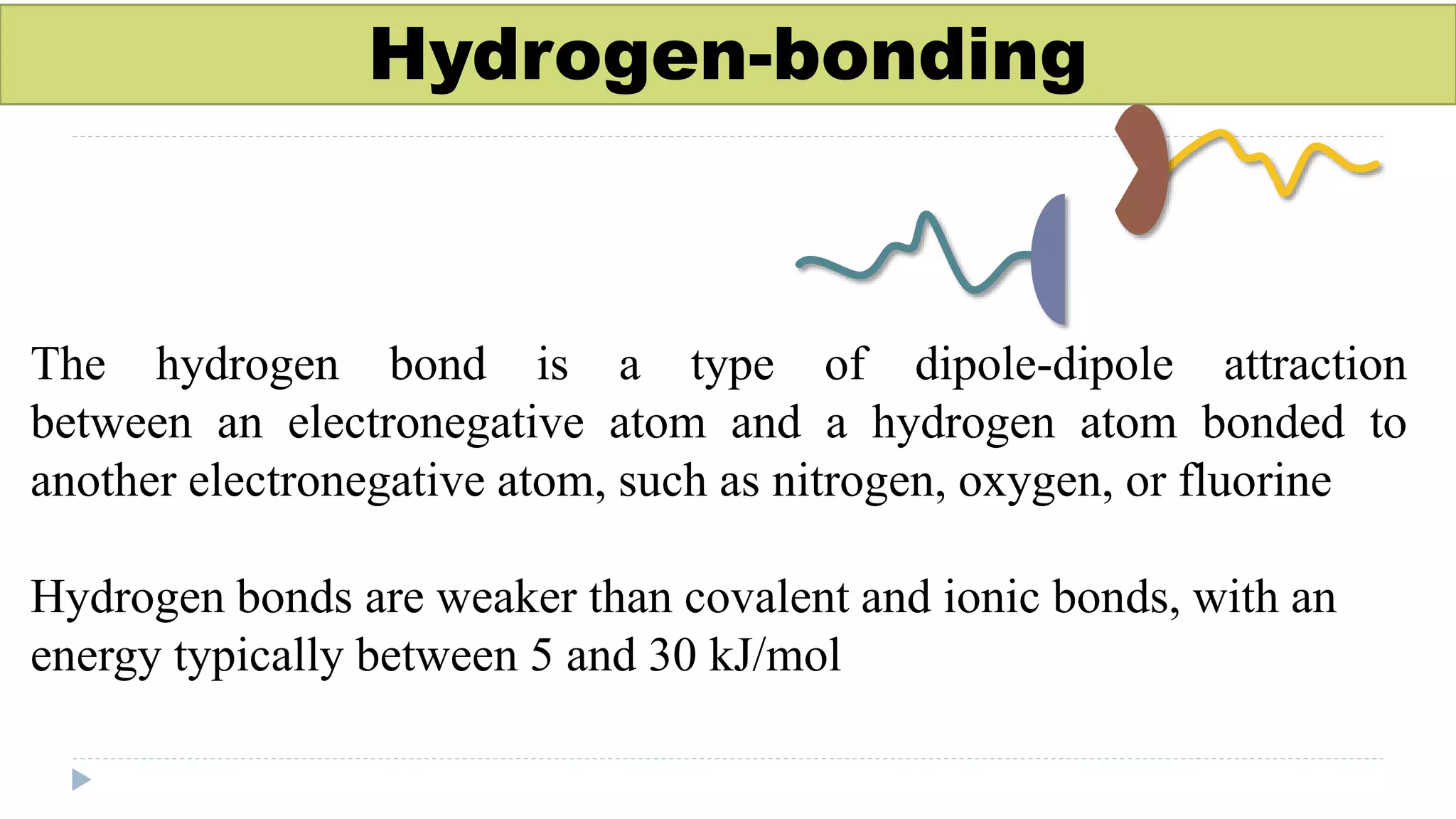
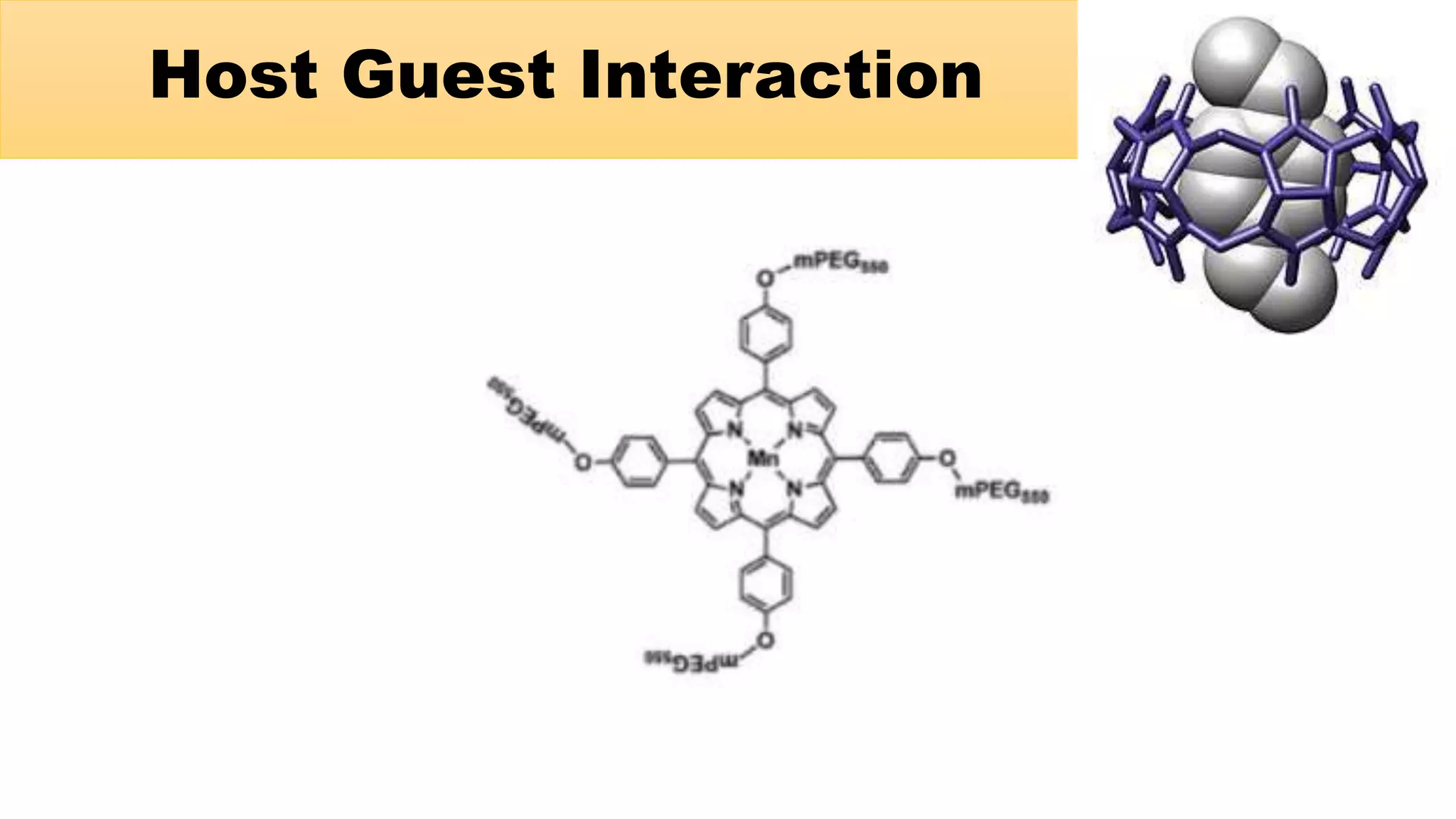
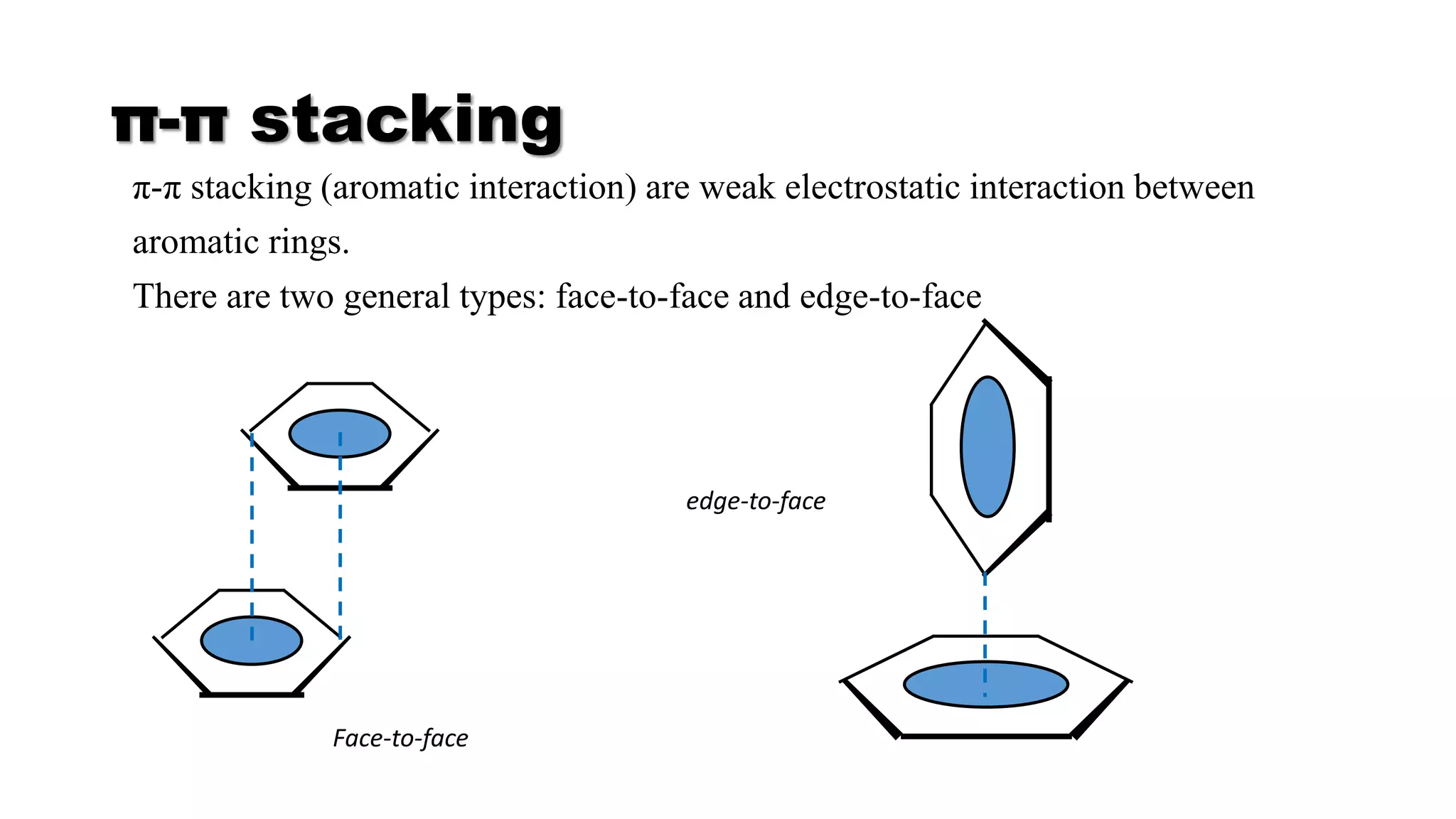
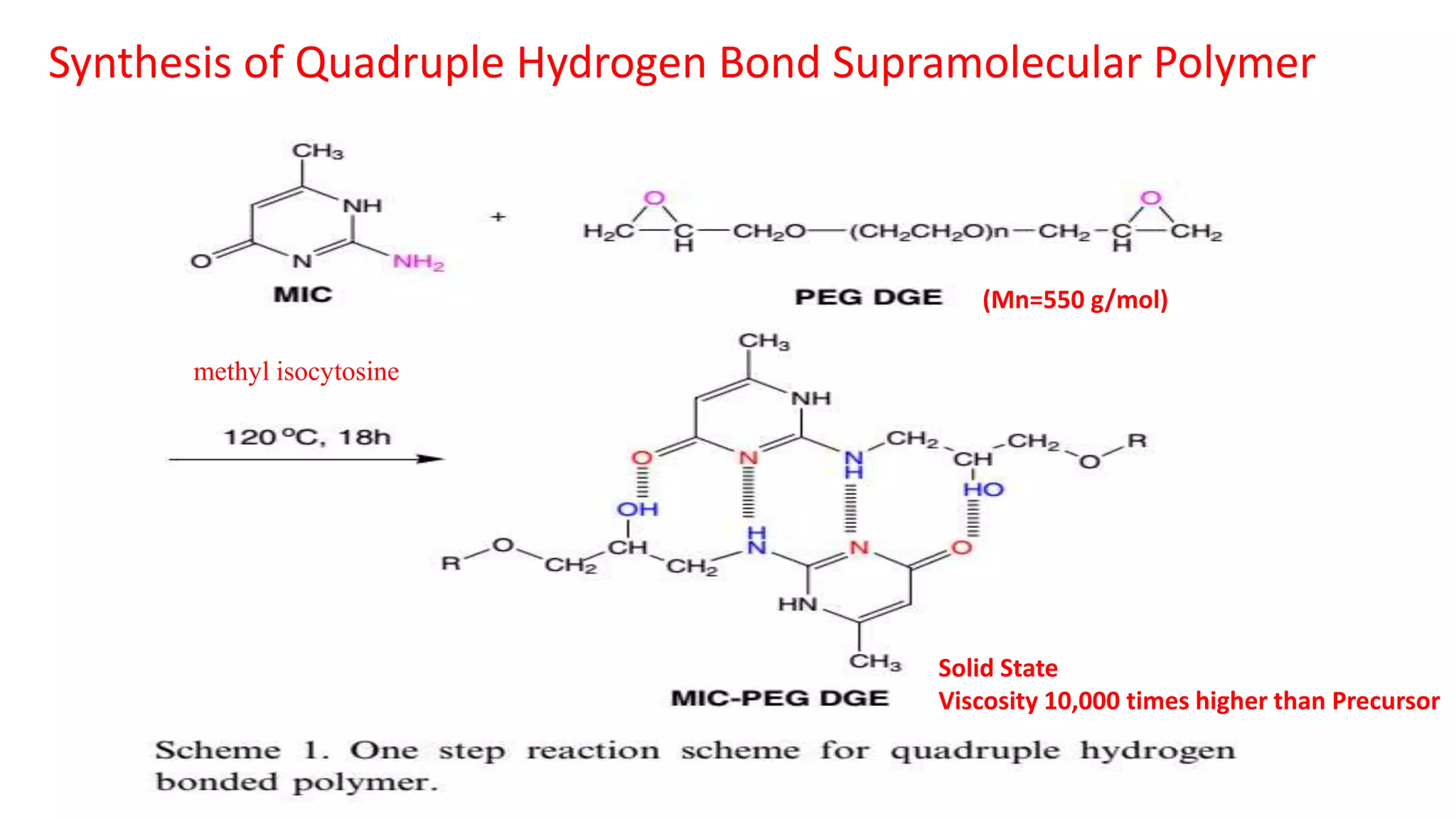
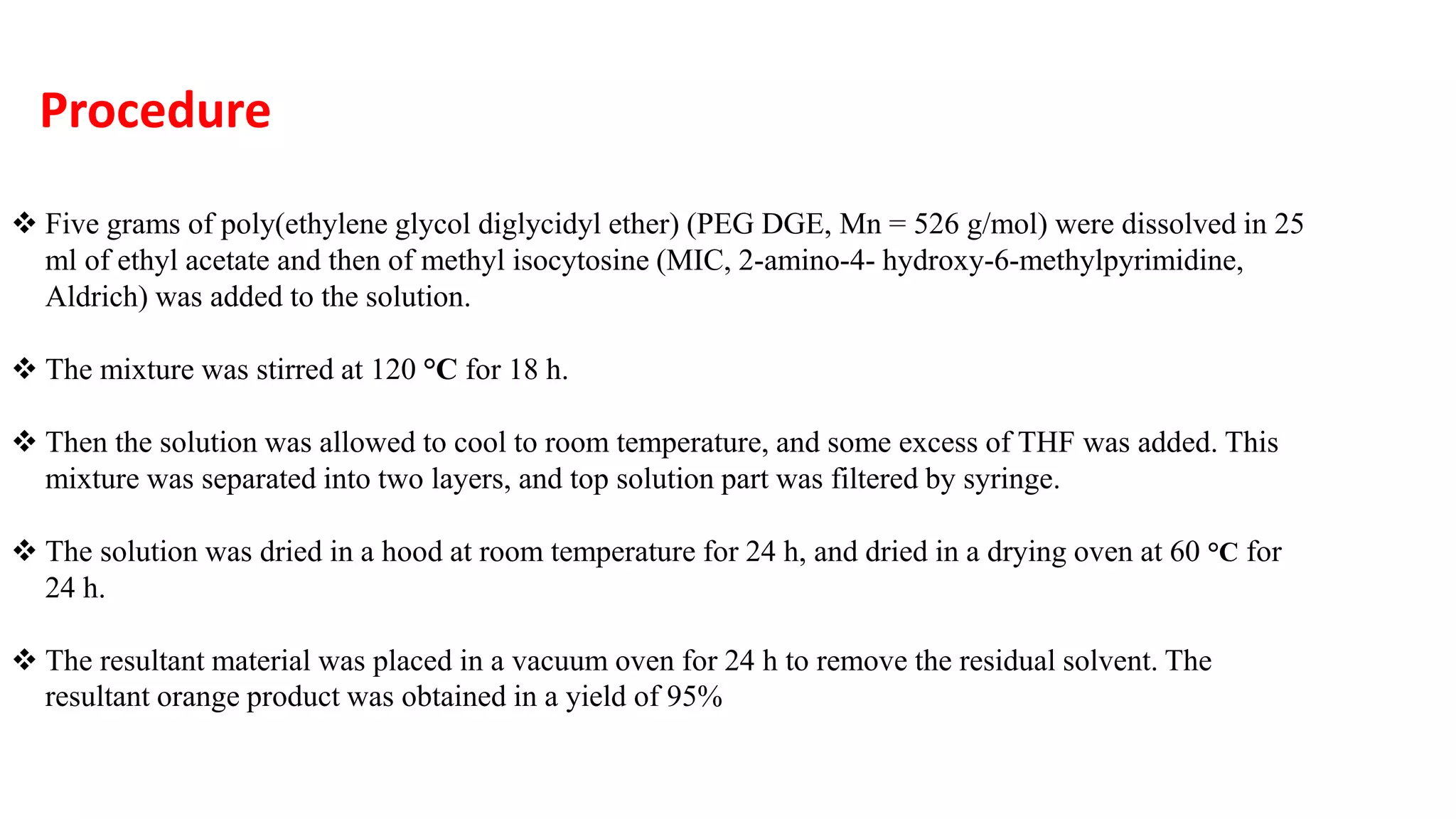
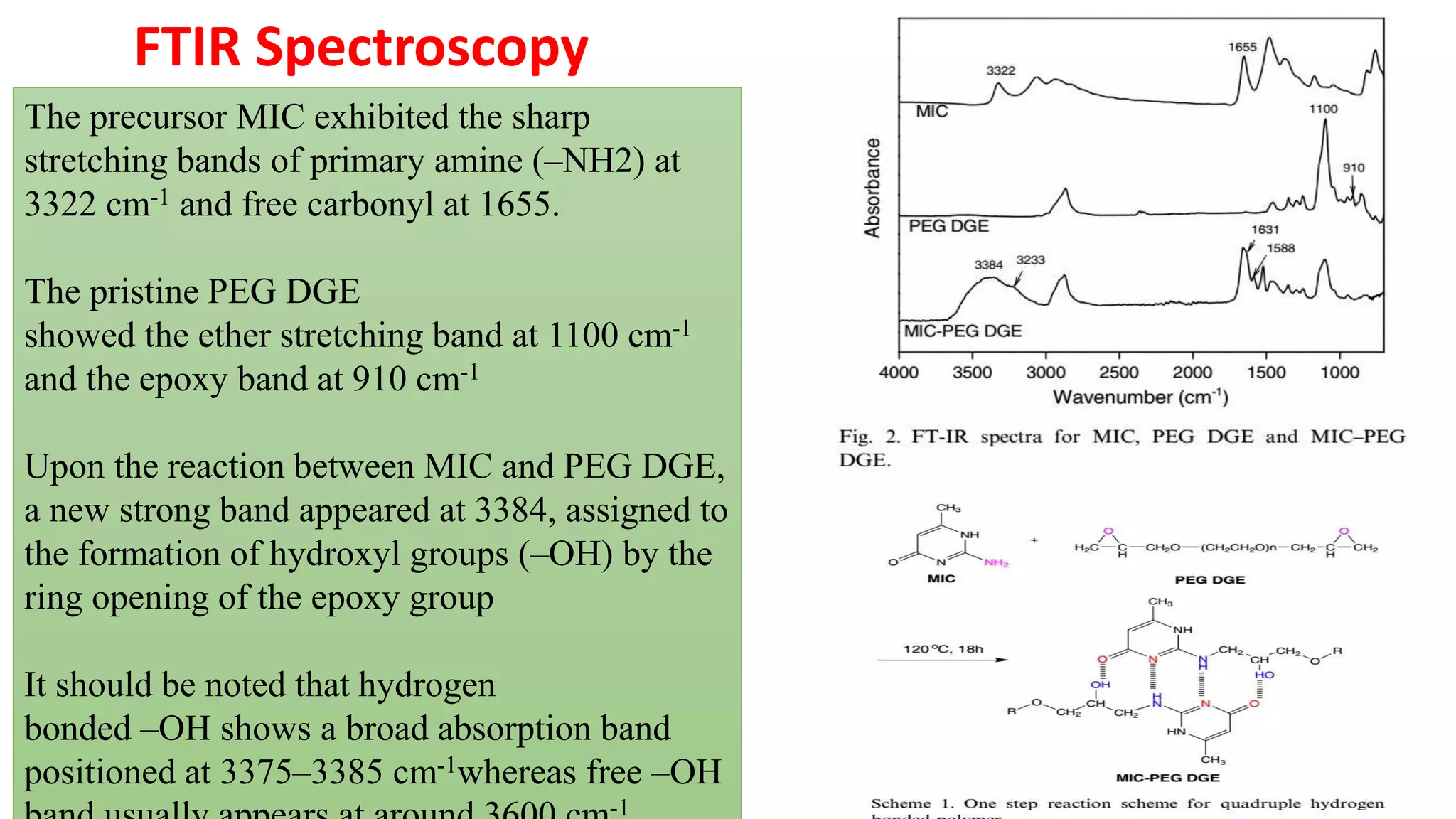
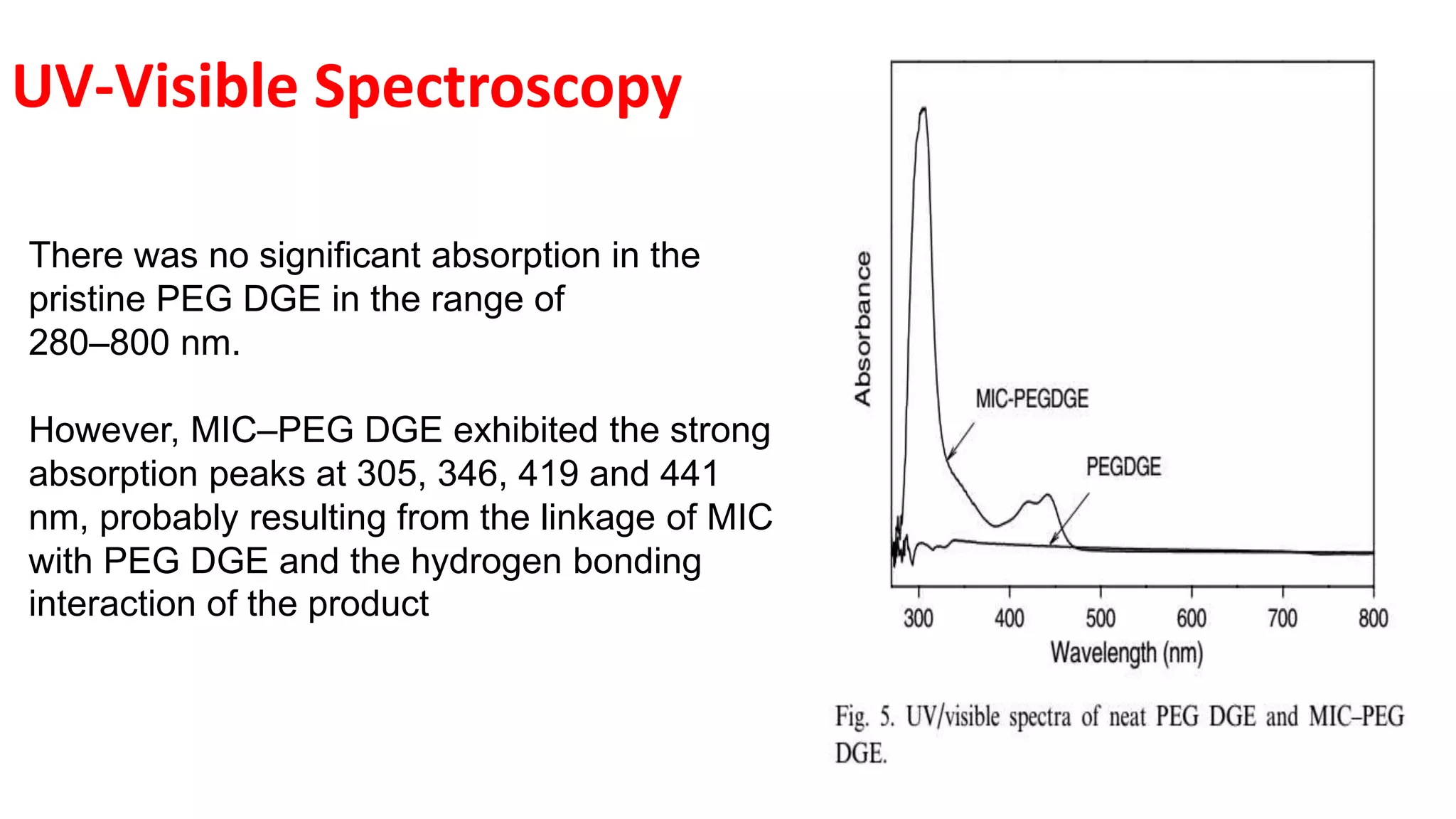
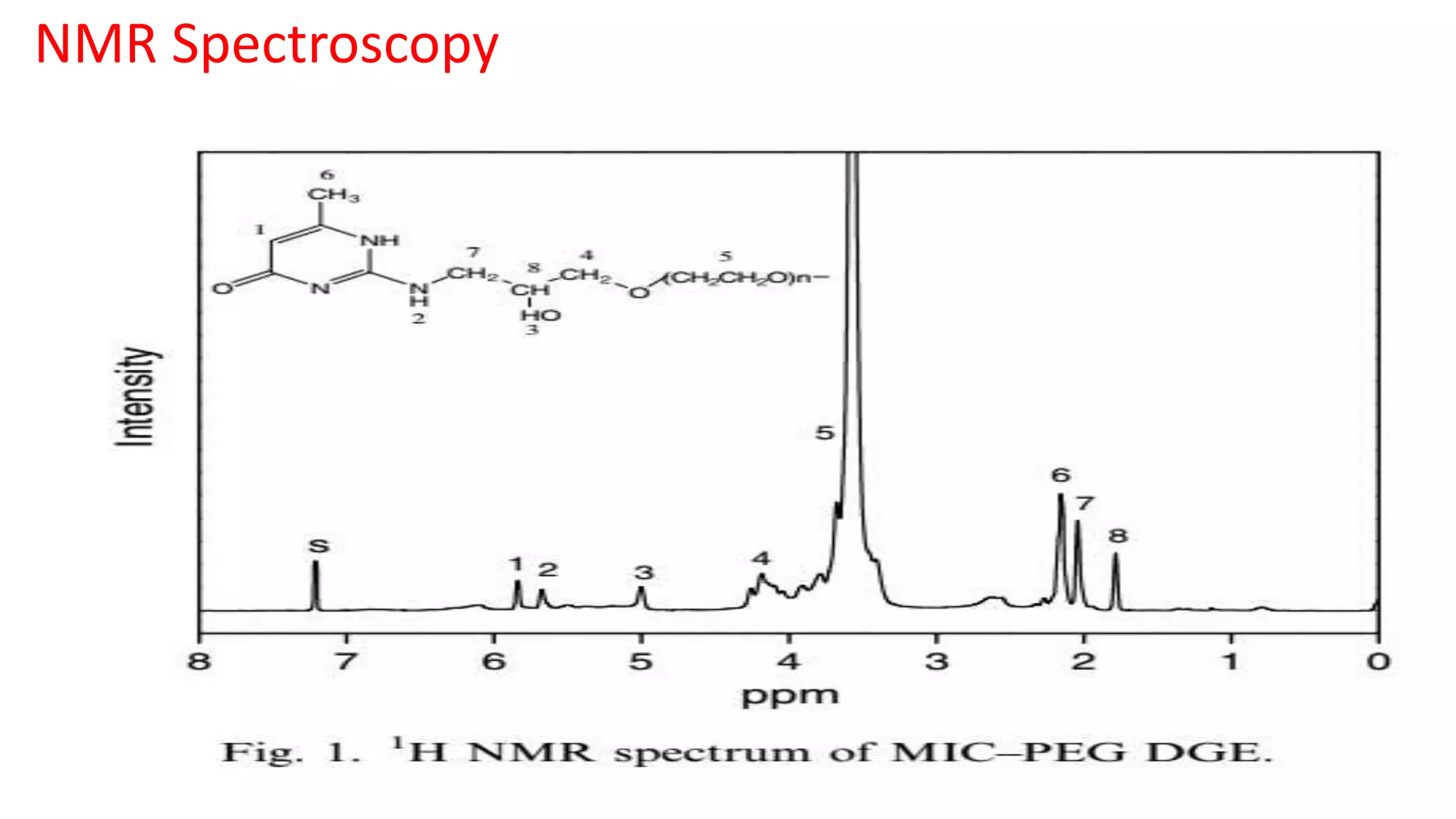
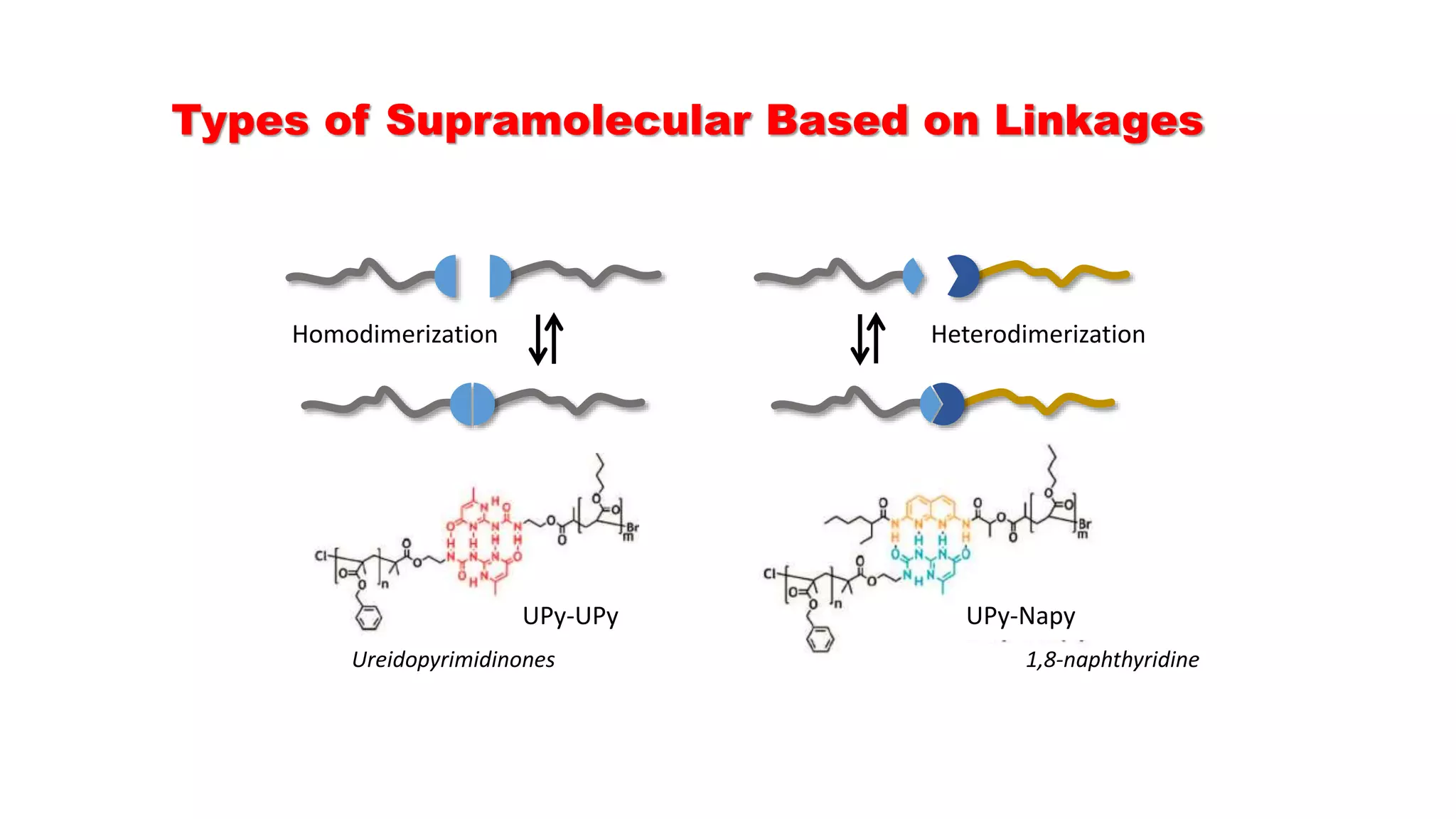
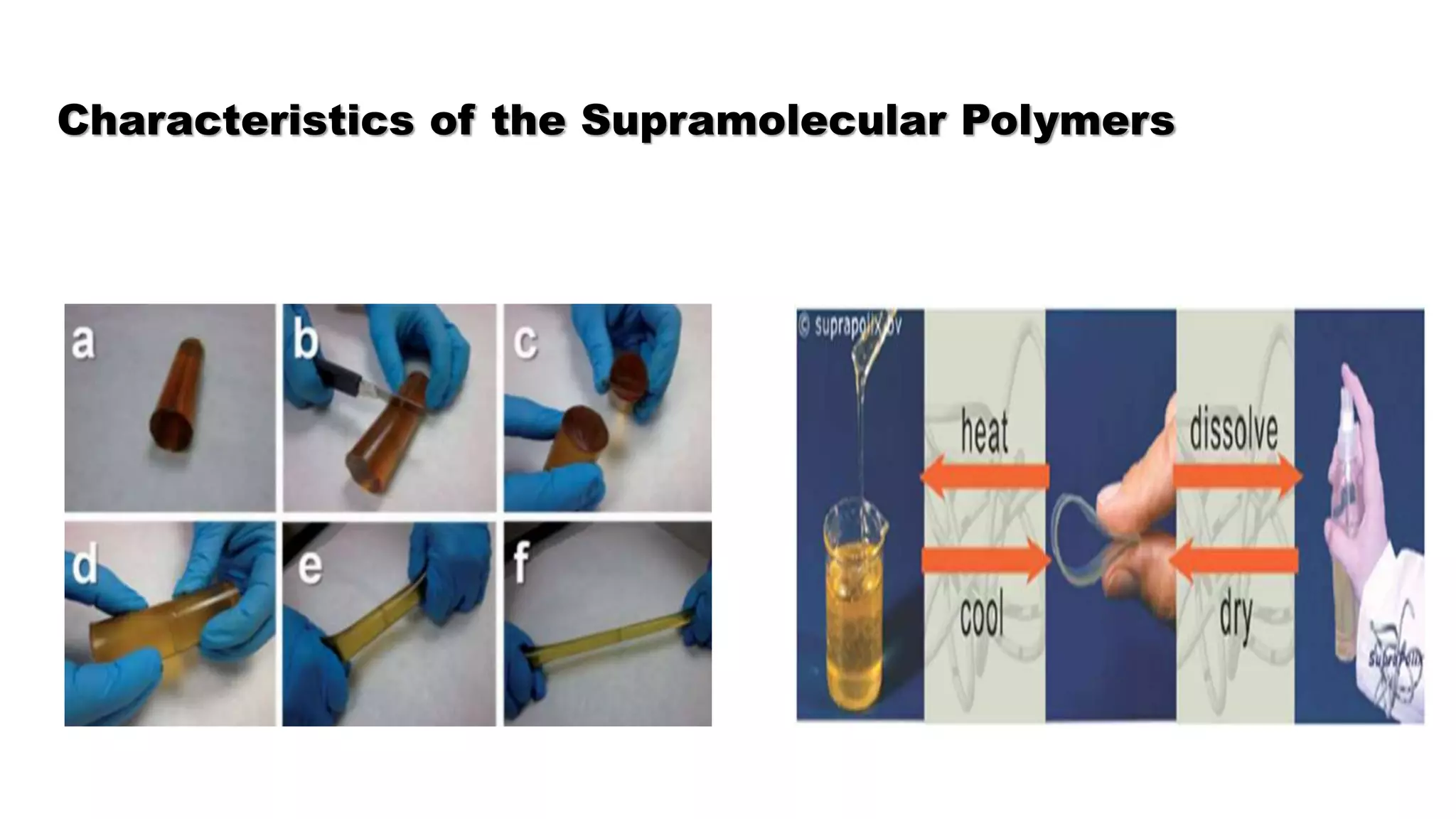
This document discusses supramolecular polymers, which are polymeric arrays brought together by non-covalent interactions like hydrogen bonding, metal coordination, and π-π stacking. It provides examples of hydrogen bonding between molecules like PEG and methyl isocytosine, and metal coordination involving ligands. Supramolecular polymers can be synthesized through reactions like one producing a quadruple hydrogen bond supramolecular polymer from PEG and MIC, with characteristics analyzed using techniques like FTIR, UV-Vis, NMR, and DSC.