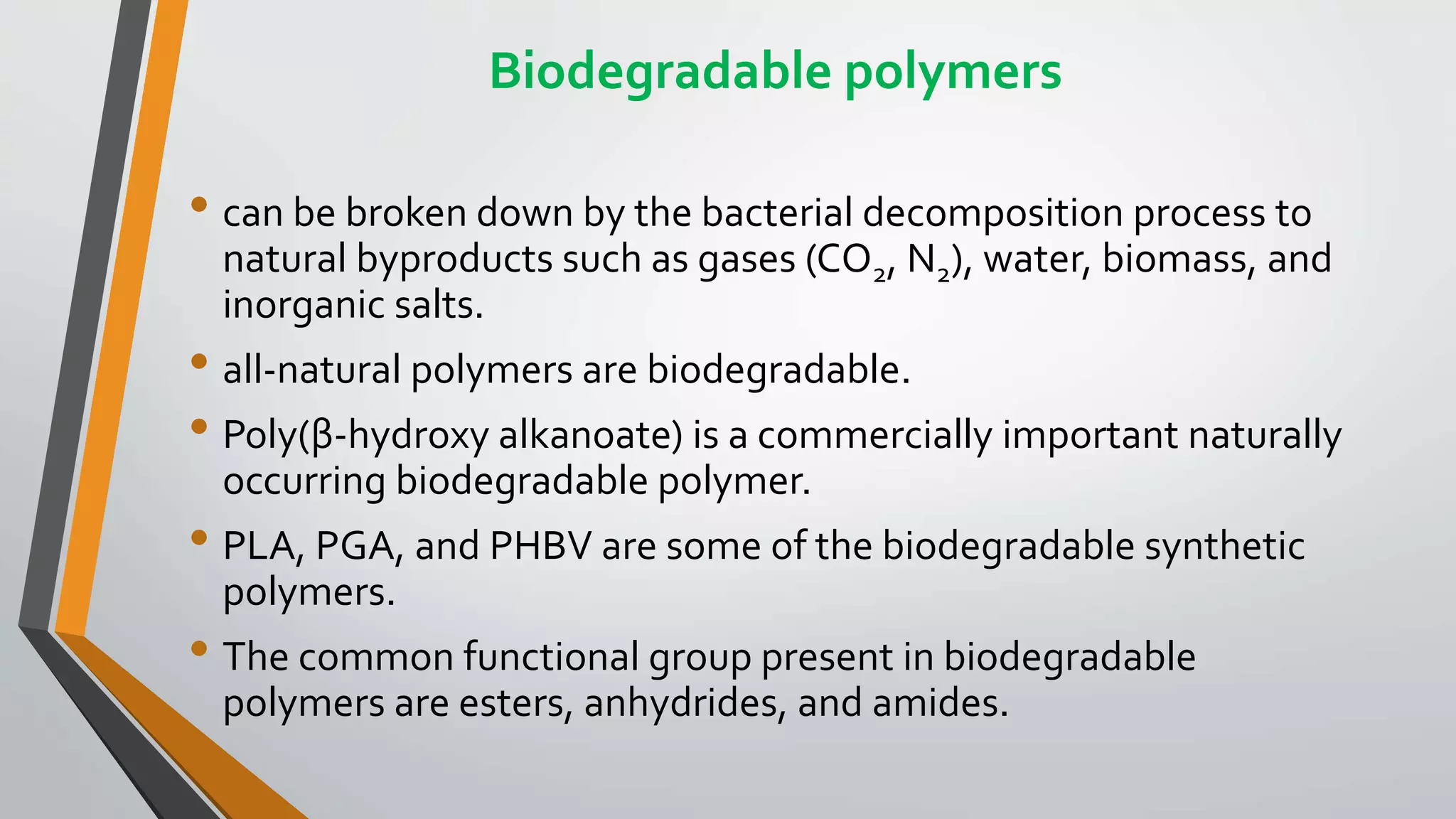
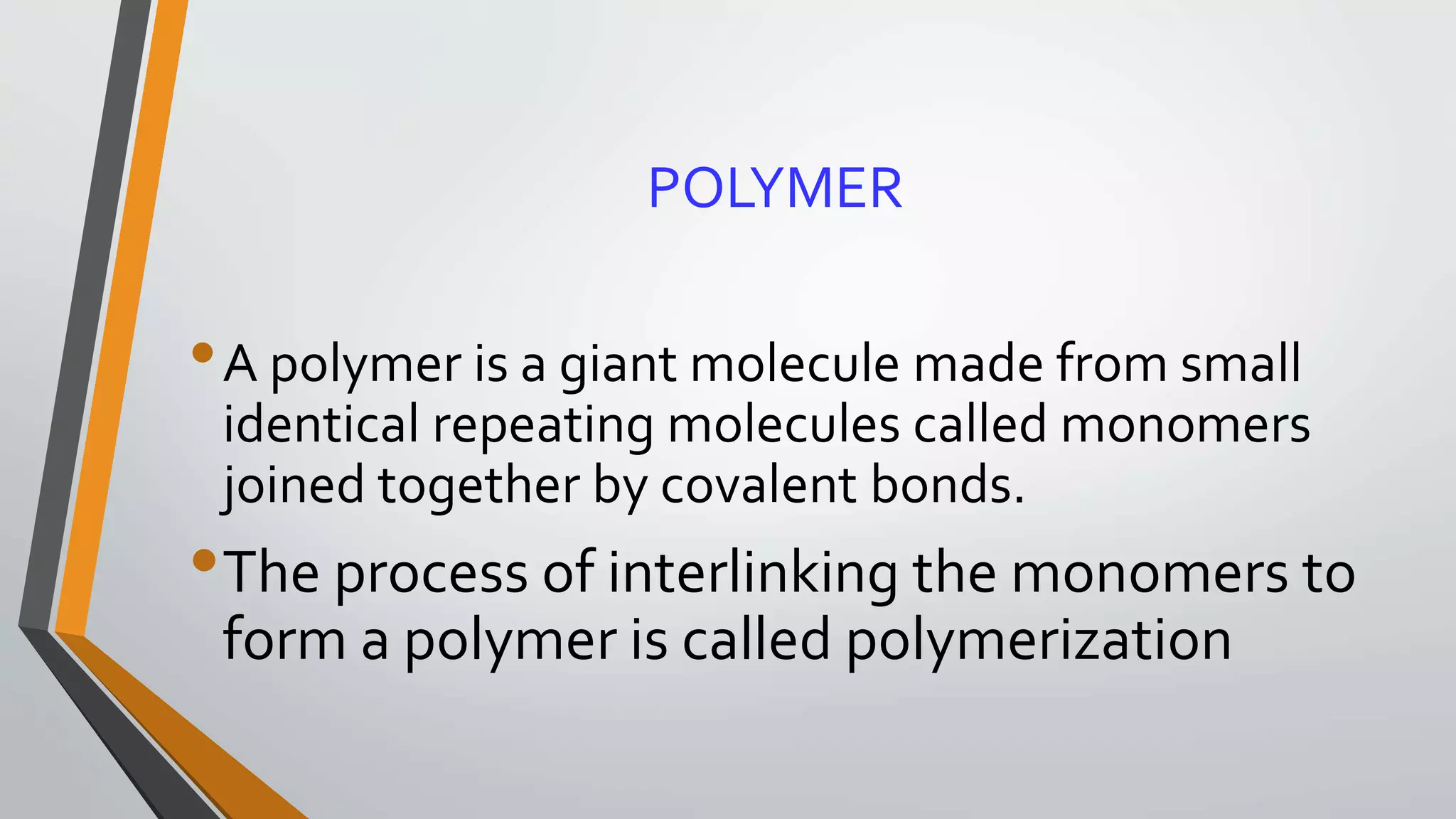
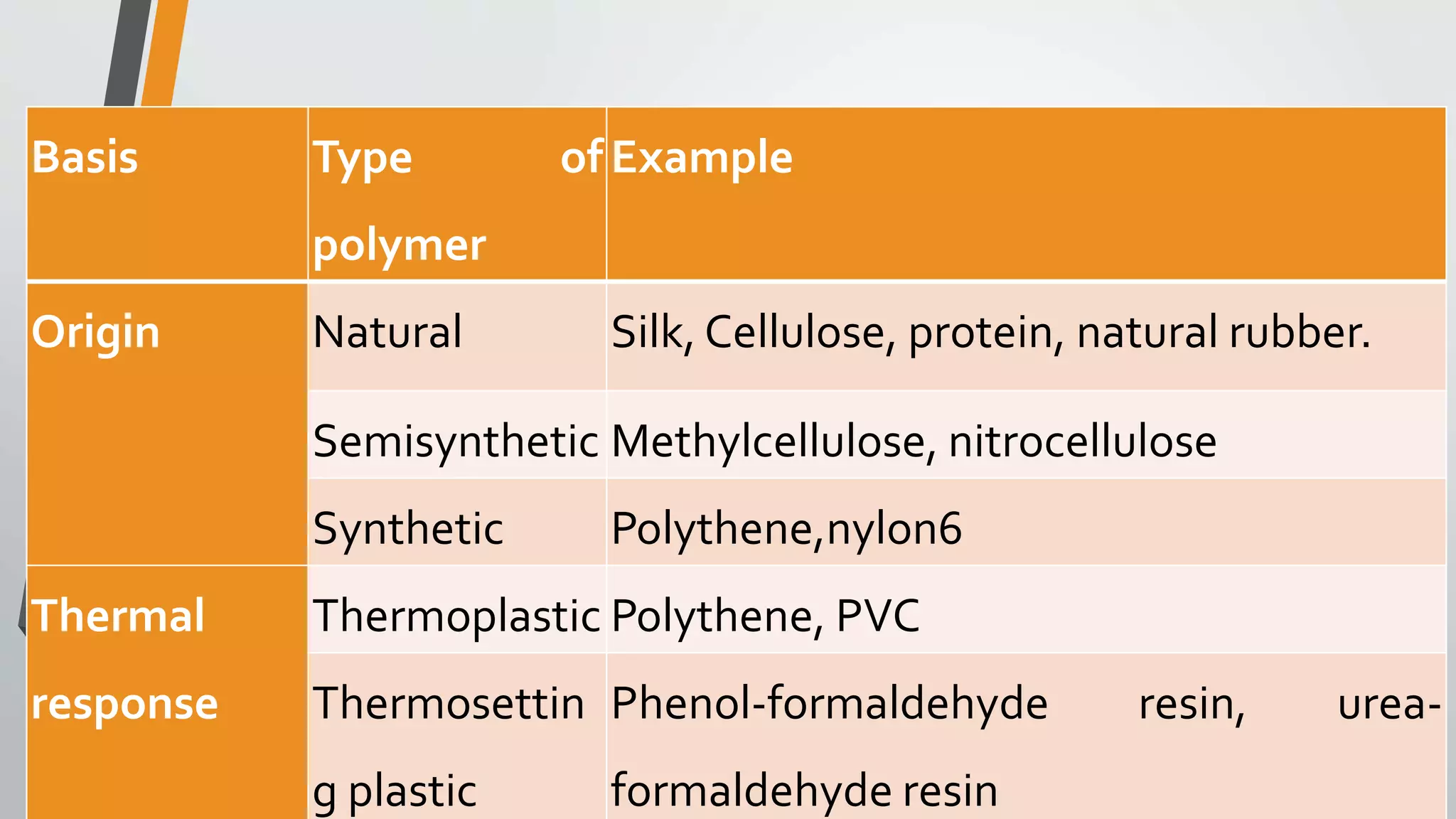
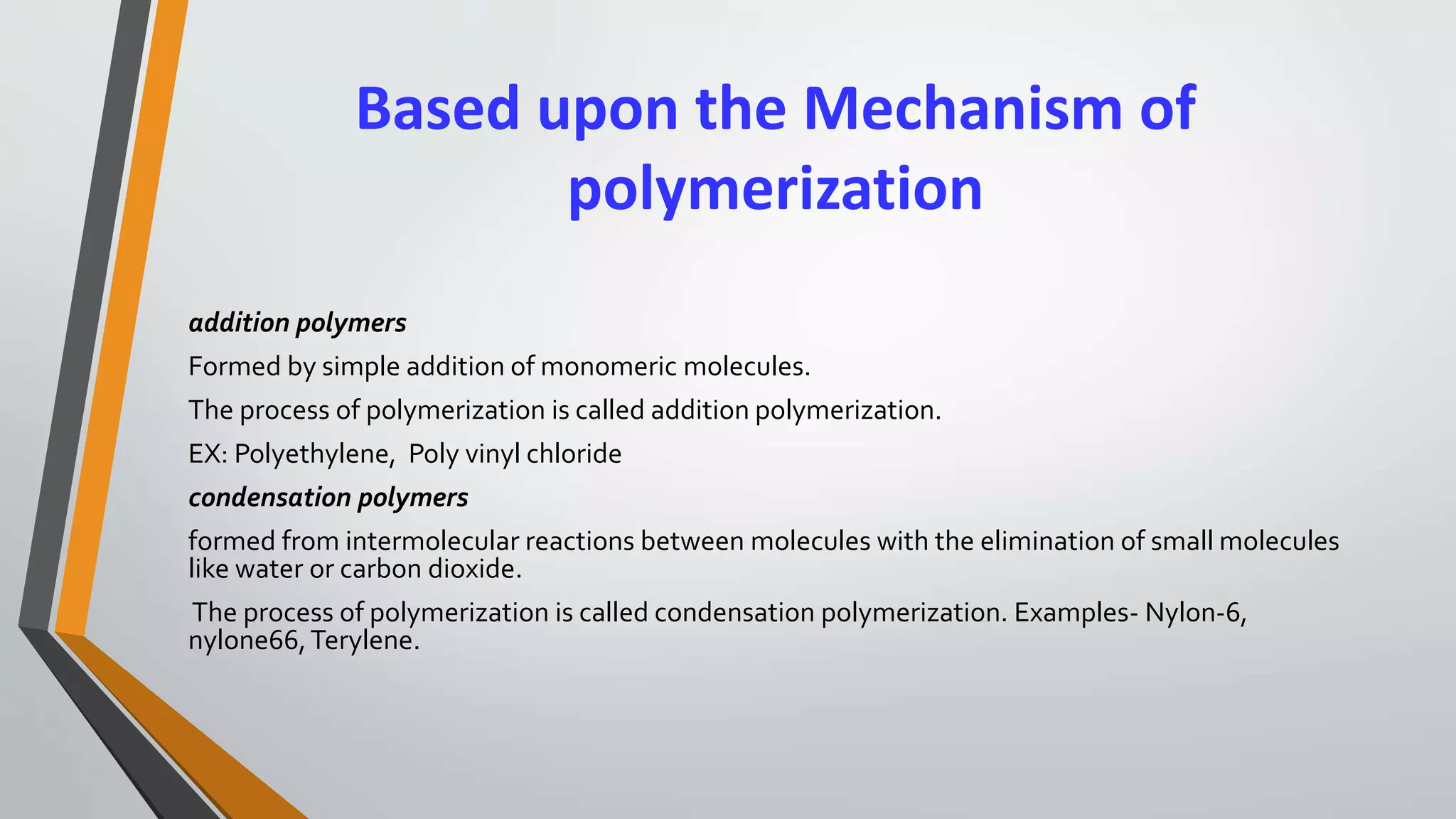
The document provides a comprehensive overview of synthetic polymers, including definitions, classifications, and examples. It discusses various polymer types based on origin, thermal response, structure, physical properties, and degradability, along with detailed information on specific polymers like polyethylene, nylon, and biodegradable options. Additionally, it explains the processes of polymerization and applications of different polymers in industry and everyday use.






























![KEVLAR- Poly(para-phenyleneterephthalimide)
• Aromatic polyamide
• Five times more tensile strength than steel
• polycondensation polymer
• Monomers are p-phenylene diamine [p-H2N-(C6H4)-NH2] and terephthaloyl
chloride. [p-ClOC-(C6H4)-COCl].](https://image.slidesharecdn.com/syntheticpolymers-220319162137/75/Synthetic-polymers-a-content-written-by-Dr-Lali-Thomas-Kotturan-about-man-made-polymers-This-covers-module-4-of-sem-6-of-UG-Chemistry-of-Calicut-University-Kerala-38-2048.jpg)