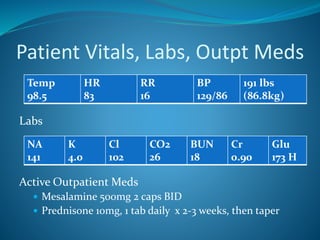
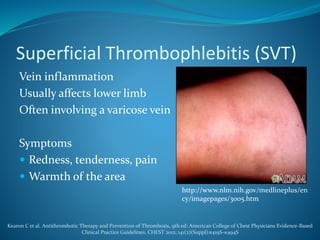
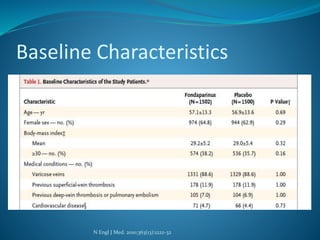
The document discusses a patient with left leg pain and erythema, along with a history of Crohn's disease, presenting a differential diagnosis primarily focused on superficial vein thrombosis (SVT) and its treatment options. Key findings highlight the effectiveness of fondaparinux in reducing thromboembolic complications compared to placebo, with considerations on treatment duration and pharmacotherapy recommendations. The pharmacist's role includes patient education on medication administration, potential side effects, and the importance of compliance with treatment protocols.