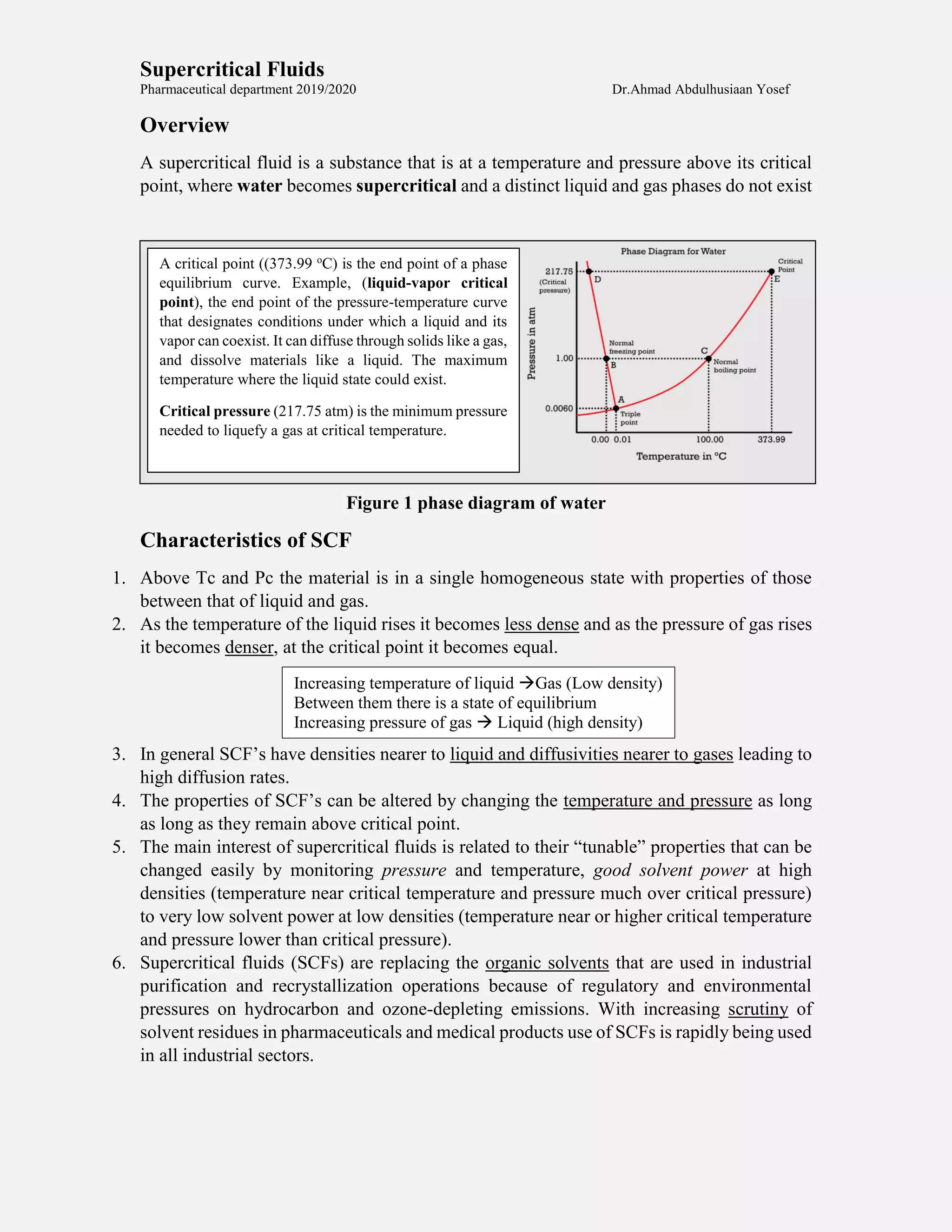
Supercritical fluids are substances above their critical point where distinct liquid and gas phases do not exist. They have densities nearer to liquids and diffusivities nearer to gases. Their properties can be tuned by adjusting pressure and temperature. Supercritical fluids like carbon dioxide are replacing organic solvents in industrial purification due to their environmental benefits. They are used in extraction, particle formation, and drug delivery due to their ability to dissolve materials like liquids while diffusing through solids like gases.