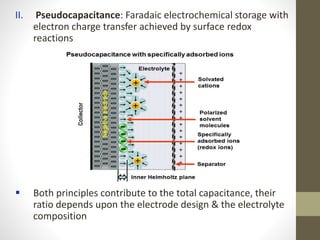
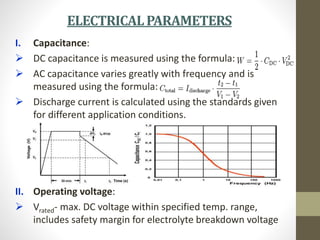
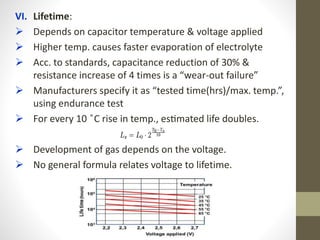
The document provides a comprehensive overview of supercapacitors, their evolution, principles, types, construction, electrical parameters, standards, applications, and market opportunities. Supercapacitors are energy storage devices characterized by high energy and power density, capable of undergoing extensive charge-discharge cycles. Key applications include consumer electronics, transportation, and energy harvesting, with ongoing research aimed at enhancing their performance and reducing costs.