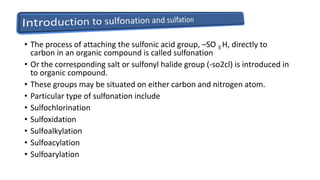
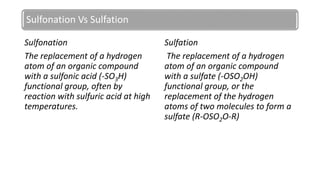
Sulfonation and sulfation are industrial chemical processes used to make products like dyes, pigments, and detergents. Sulfonation involves attaching a sulfonic acid group (-SO3H) to an organic compound, often using sulfuric acid at high temperatures. Sulfation attaches a sulfate group (-OSO2OH) or forms a sulfate bridge between two carbon atoms. These reactions are important industrially but difficult to perform on a large scale due to the exothermic and rapid reaction of SO3. Over 1.6 million metric tons of sulfonates and sulfates are produced annually, primarily for use as surfactants in laundry and cleaning products.




![• Sulfonation and sulfation are major industrial chemical processes
used to make a diverse range of products, including dyes and color
intensifiers, pigments, medicinals, pesticides and organic
intermediates. Additionally, almost 500,000 metric tons per year of
lignin sulfonates are produced as a by-product from paper pulping.
Petroleum sulfonates are widely used as detergent additives in
lubricating oils. However, the majority of the 1.6 million metric tons
of sulfonates and sulfates produced annually in the United States[1]
are used as surfactants in laundry and consumer products
applications.](https://image.slidesharecdn.com/sulfonationsulfation-170501115254/85/Sulfonation-amp-sulfation-8-320.jpg)
![• SO3 is an aggressive electrophilic reagent that rapidly reacts with any
organic compound containing an electron donor group.
• Sulfonation is a difficult reaction to perform on an industrial scale
because the reaction is rapid and highly exothermic,
• Releasing approximately 380 kJ/kg SO3 (800 BTUs per pound of SO3)
reacted[2].](https://image.slidesharecdn.com/sulfonationsulfation-170501115254/85/Sulfonation-amp-sulfation-9-320.jpg)
