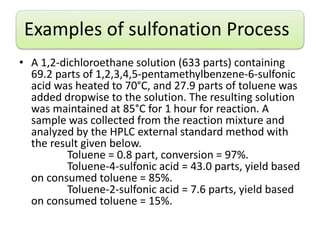
This document discusses sulfonation agents and processes. It describes problems with conventional sulfonating agents like sulfuric acid and outlines an improved sulfonation process using substituted methylbenzene sulfonic acids as sulfonating agents. Examples are provided of sulfonating aromatic compounds like toluene and anilines using substituted methylbenzene sulfonic acids like 1,3,5-trimethylbenzene-2-sulfonic acid in solvents like dichloroethane. This new process achieves high conversion rates and para-selectivity for the sulfonation reaction.