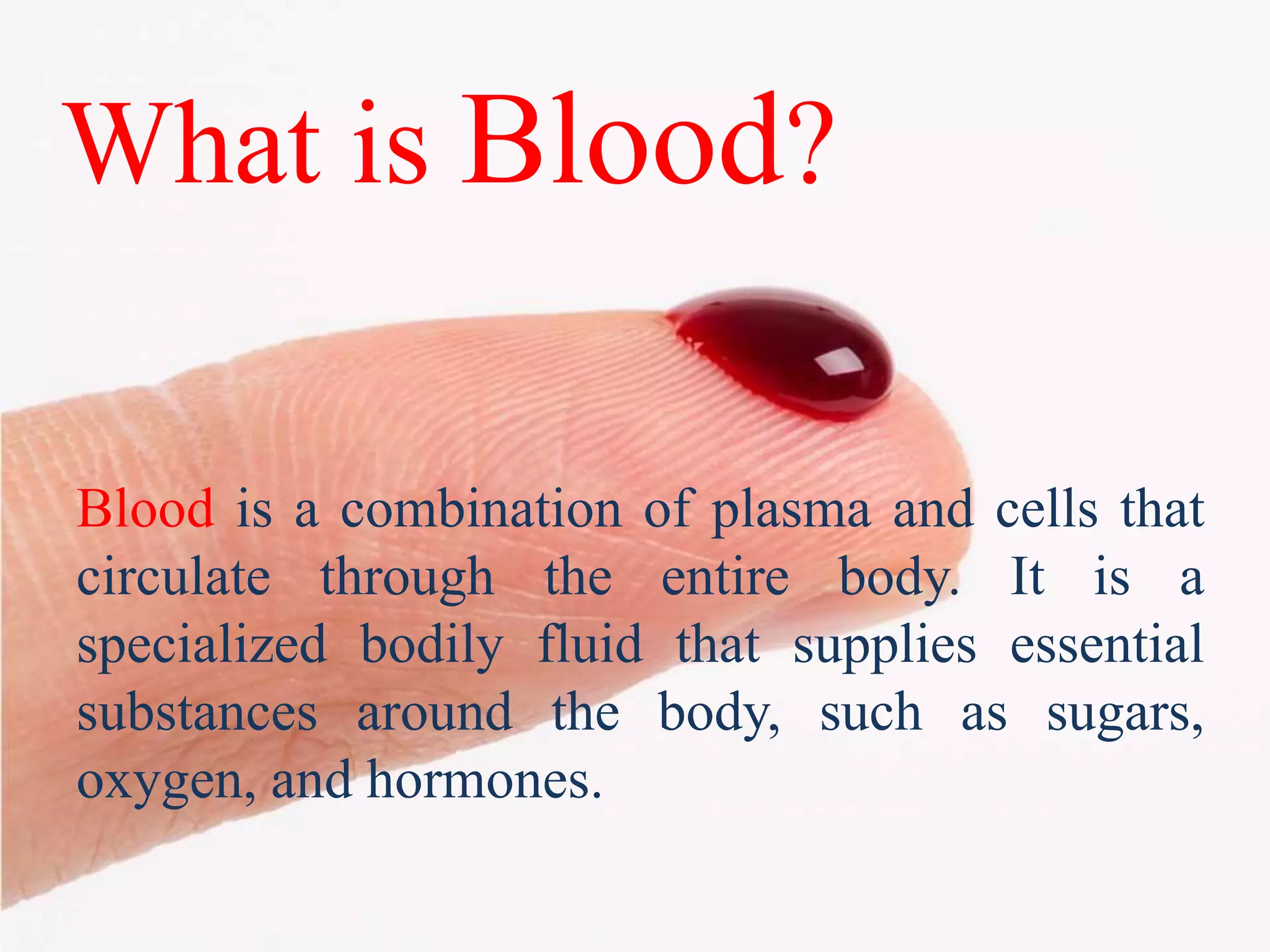
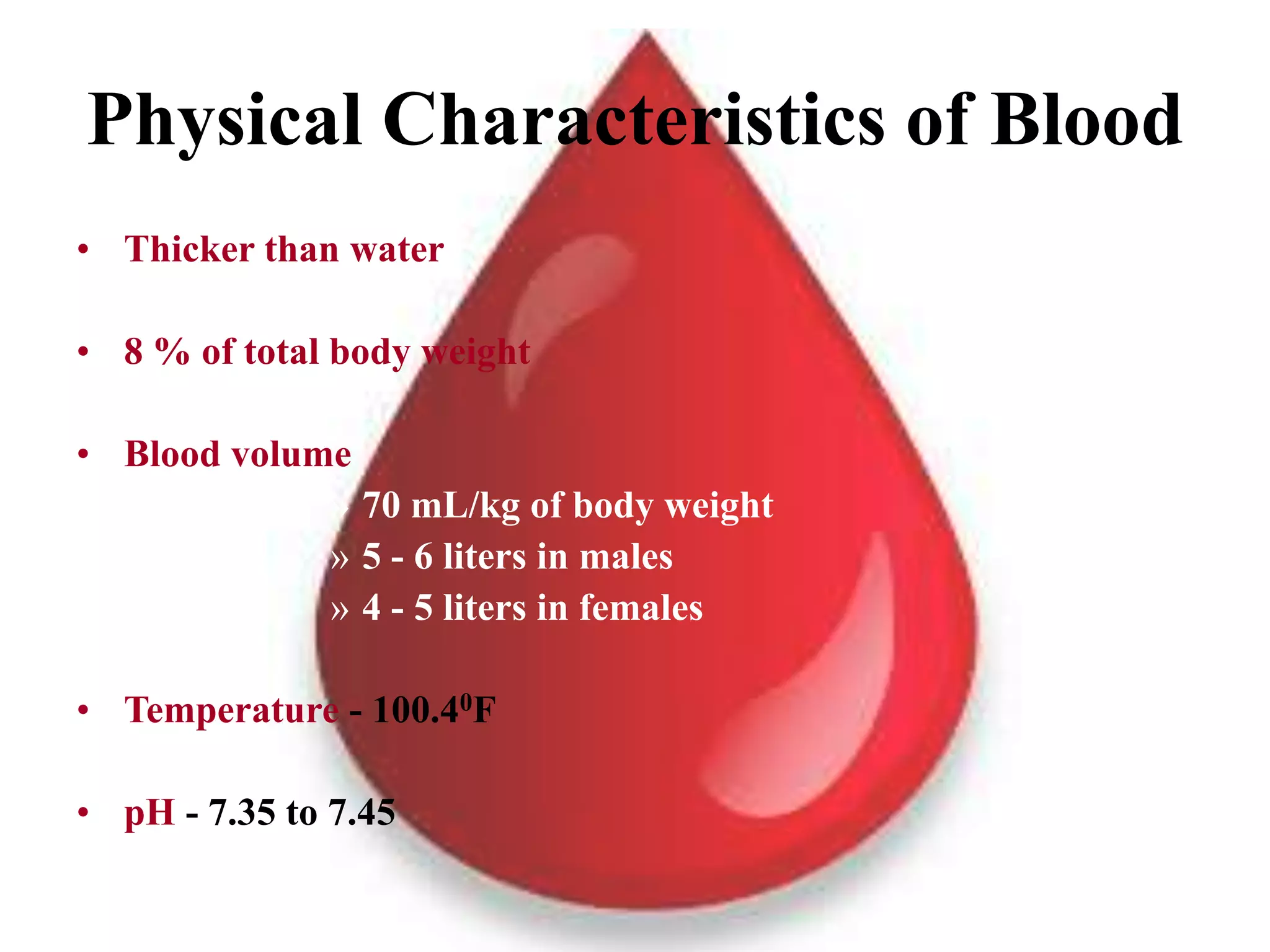
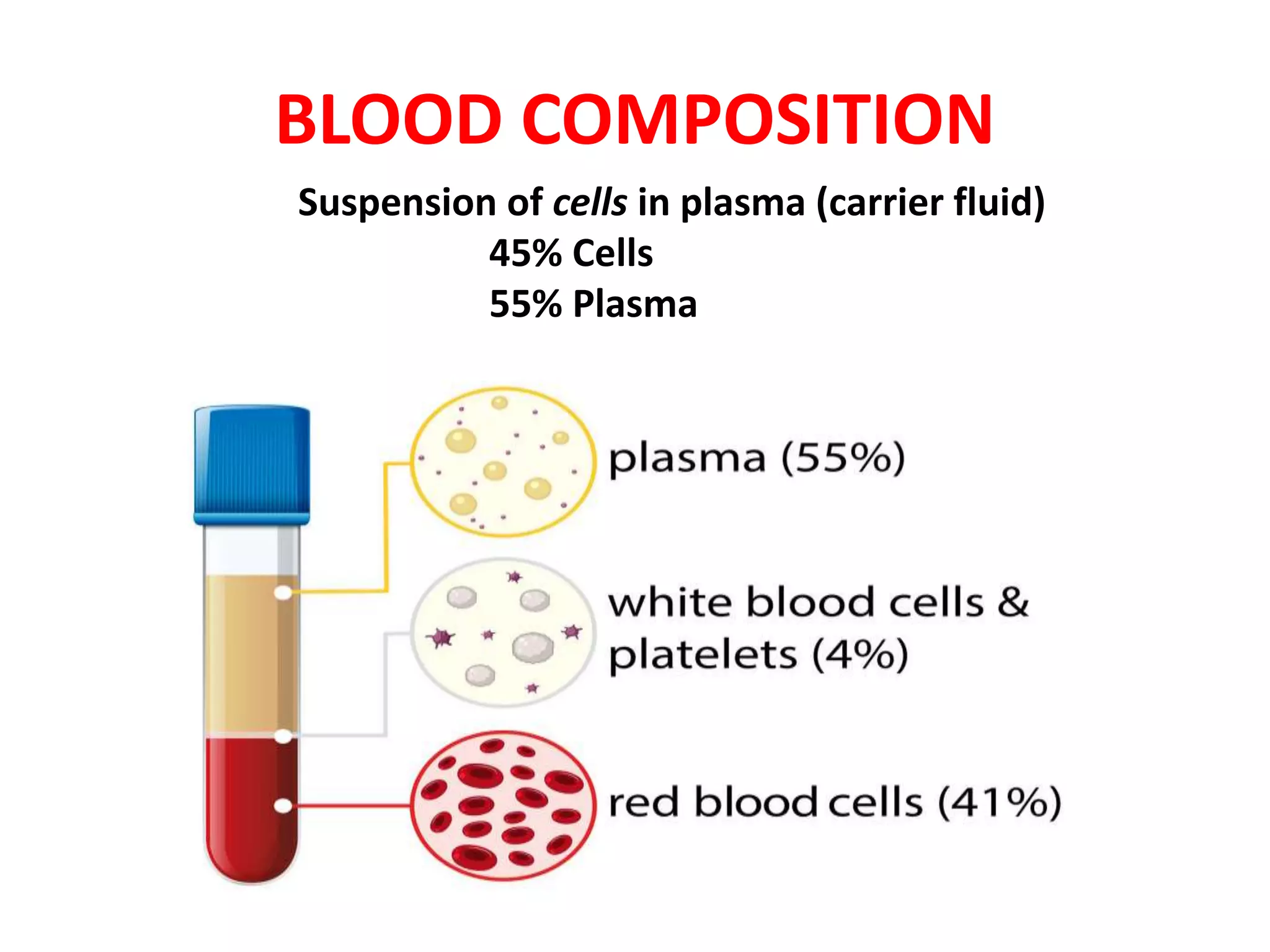
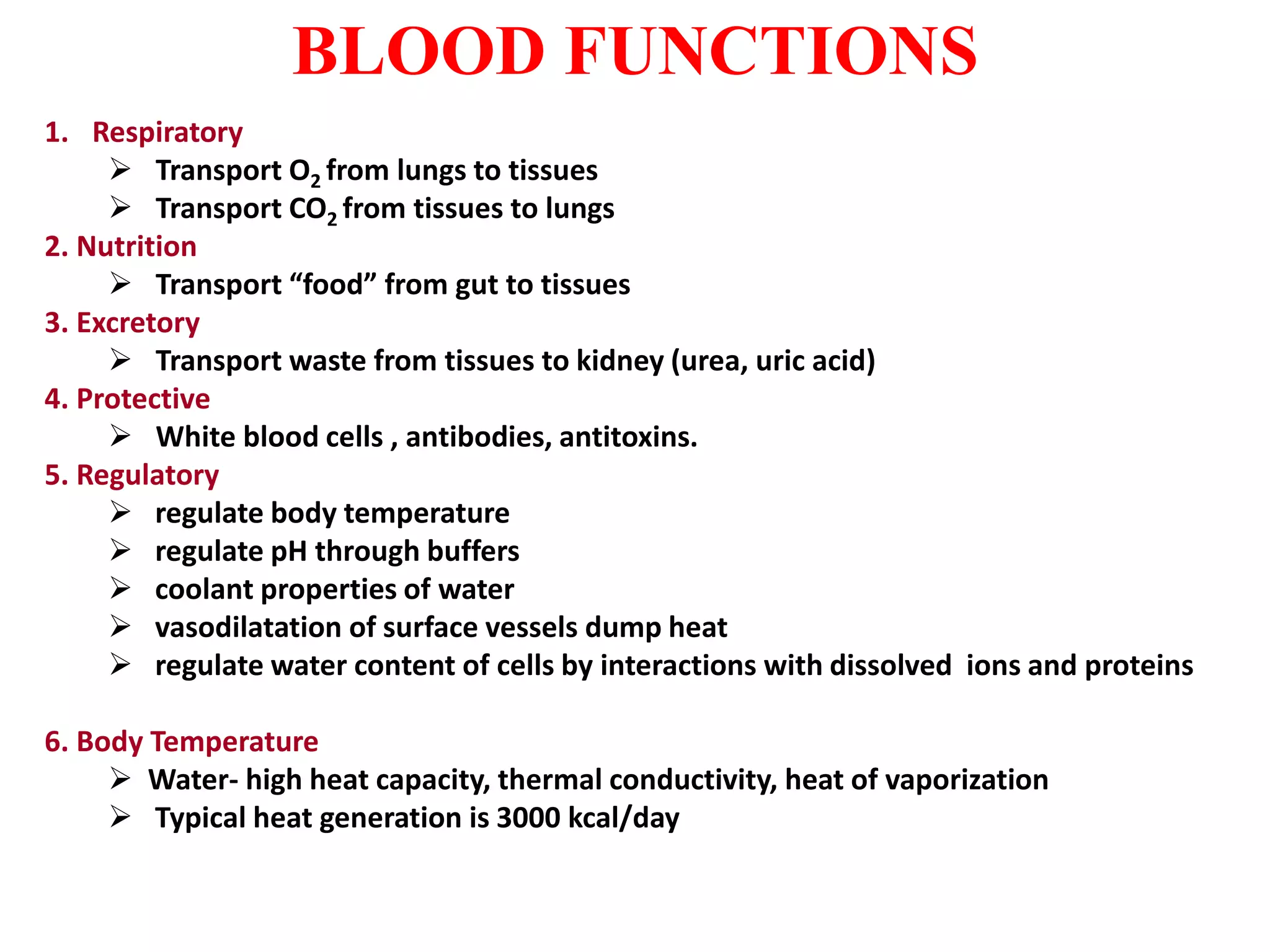
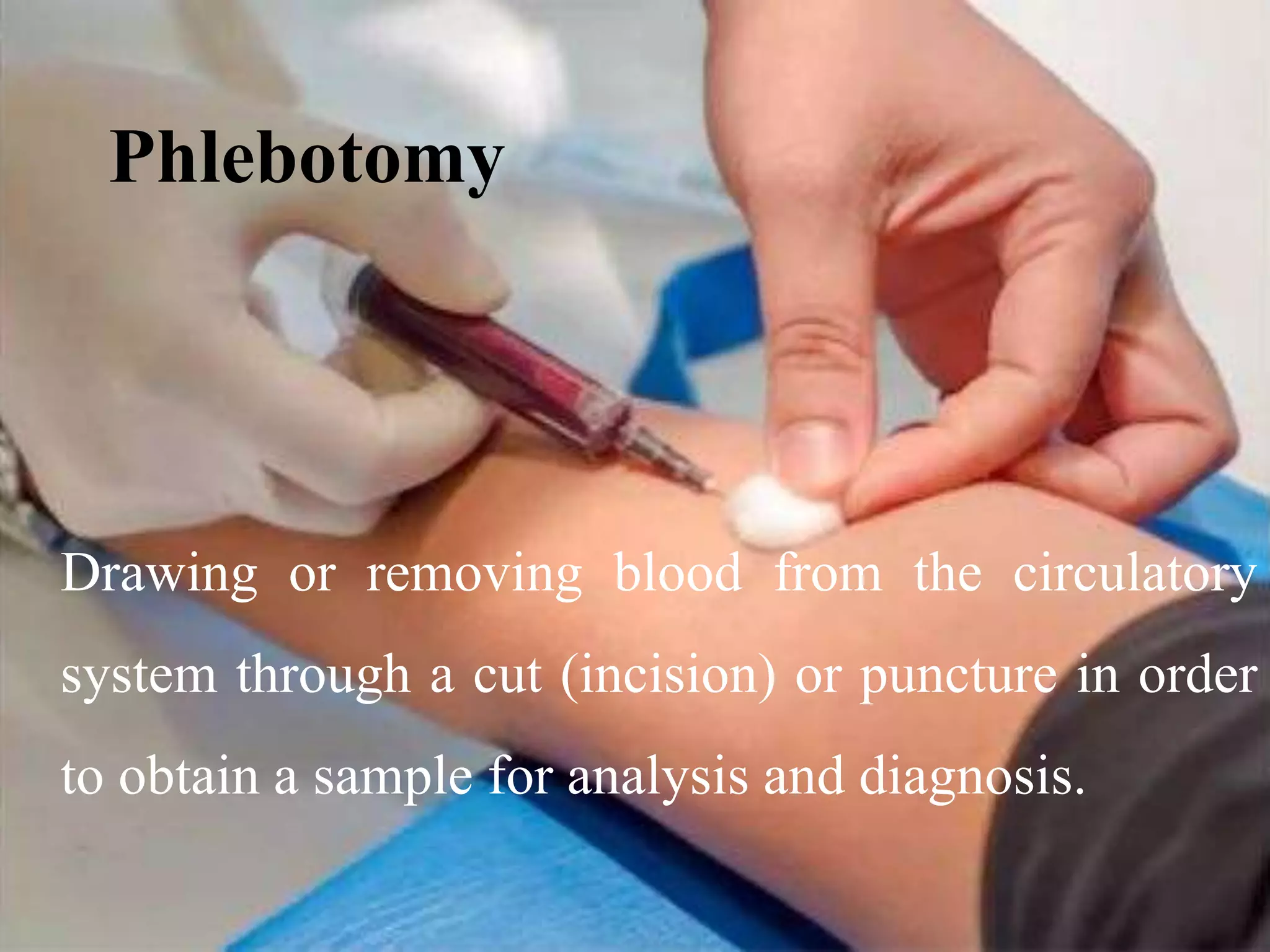
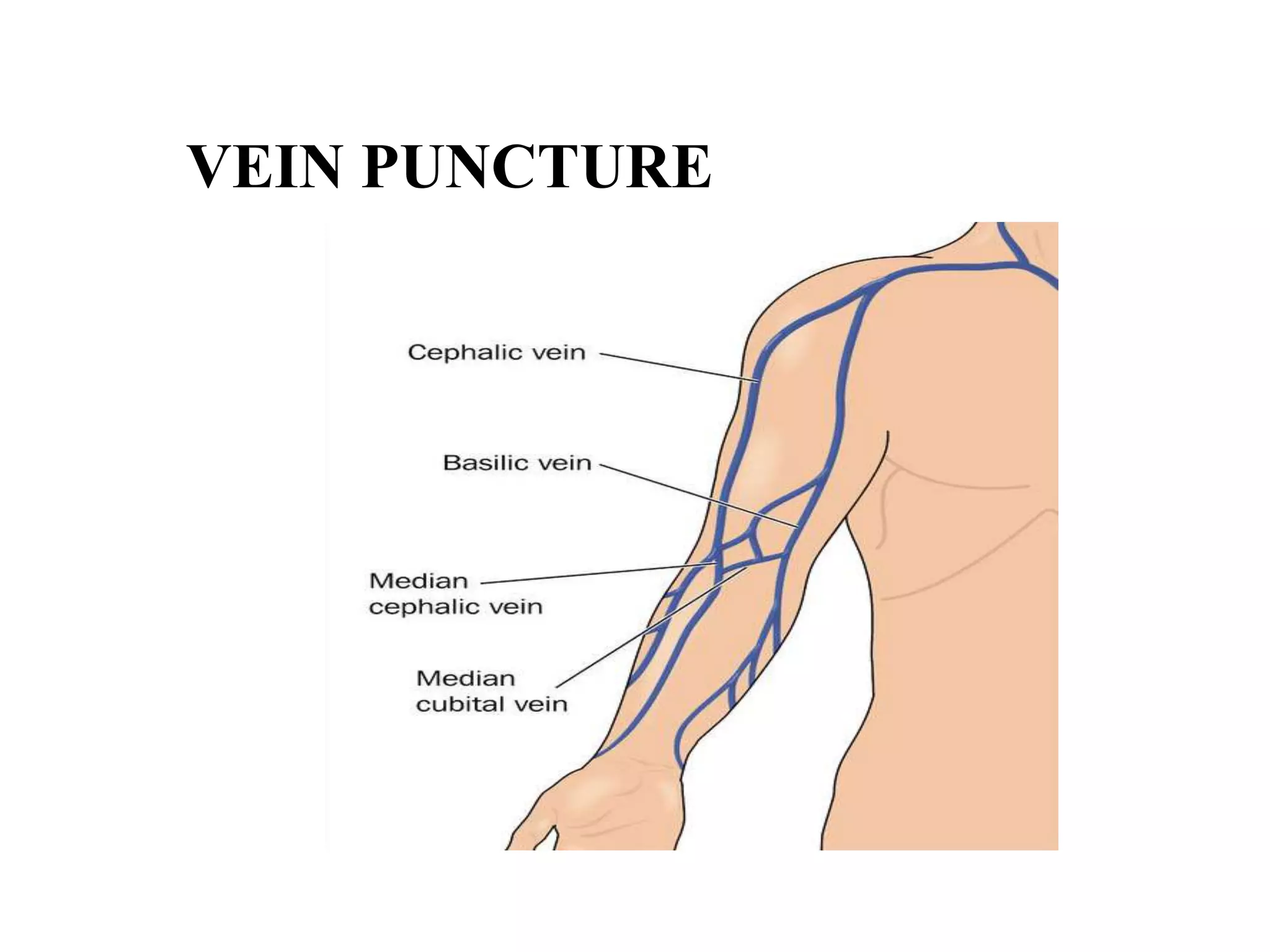
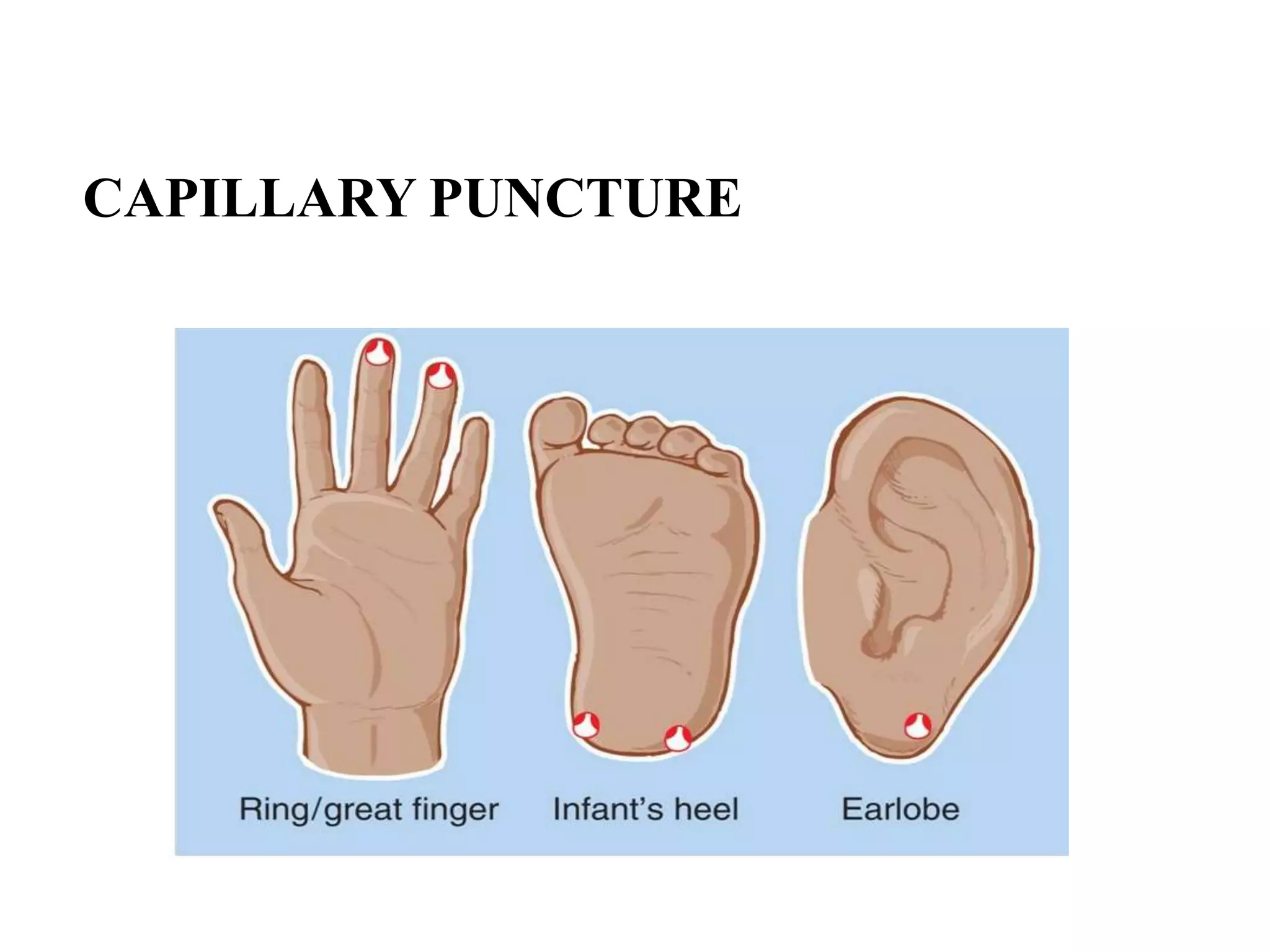
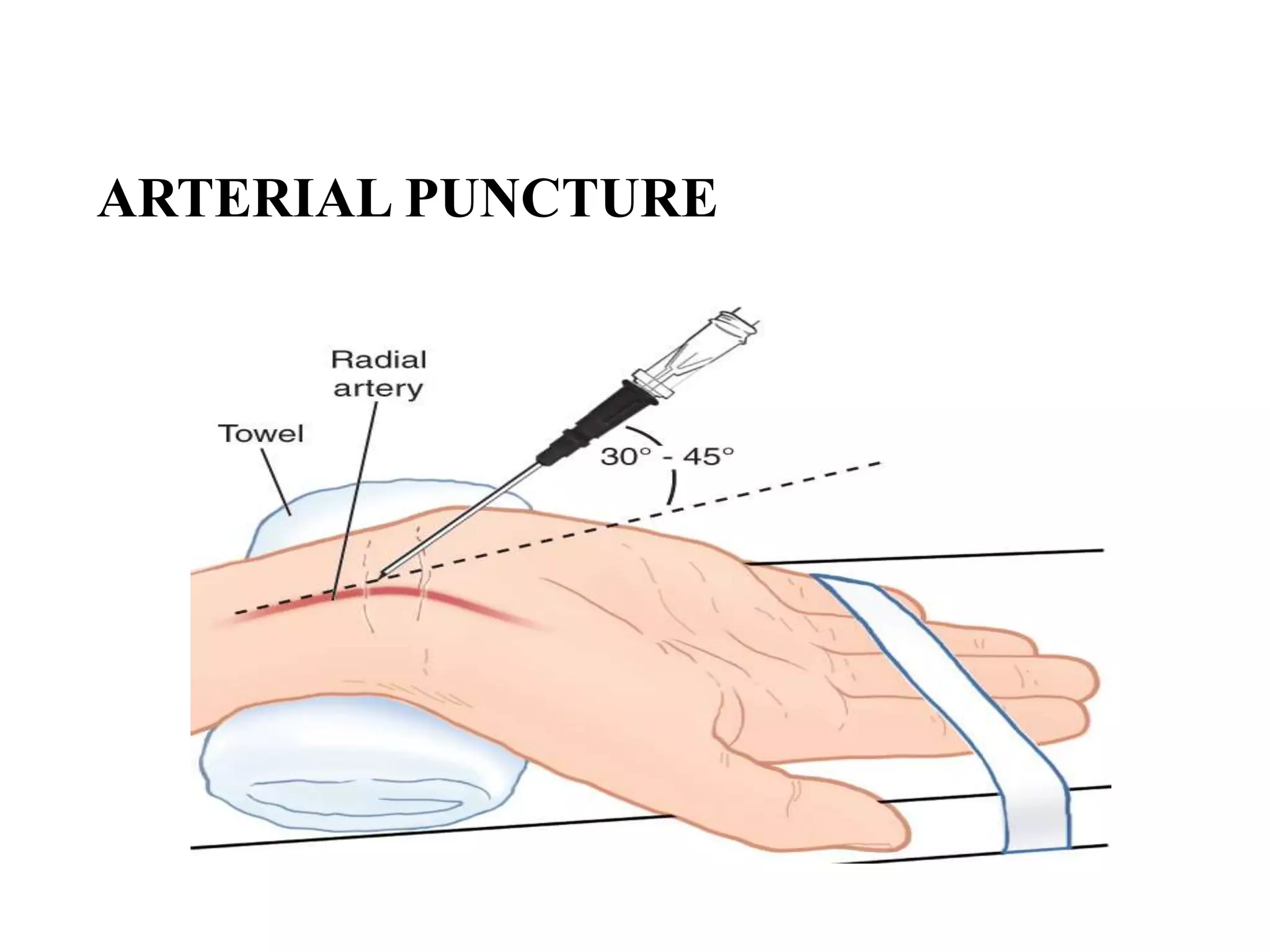
This document provides information about blood collection and processing. It defines blood and its functions. It describes the physical characteristics of blood and its composition. It discusses the purposes of blood collection and the techniques used for vein puncture, capillary puncture, and arterial puncture. It also covers sample handling, centrifugation, and factors to consider to prevent hemolysis.