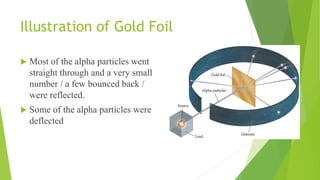
Rutherford and his colleagues conducted a gold foil experiment to test J.J. Thomson's "plum pudding" model of the atom. They bombarded a thin gold foil with alpha particles and observed that most passed through undeflected, while a small percentage were deflected or bounced straight back. This contradicted Thomson's model, where particles should experience only slight deflections, and instead supported the idea that atoms have a small, dense nucleus containing their positive charge. The experiment established Rutherford as the "father of nuclear physics" and led to the modern understanding of atomic structure.