1) Early Greek philosophers proposed theories of matter being made of elements like earth, air, fire and water. Democritus proposed atoms as the smallest indivisible units of matter in the 5th century BC.
2) In the early 1800s, John Dalton proposed atoms as the fundamental units of elements based on experimentation. He suggested atoms of each element are identical and atoms combine in fixed ratios.
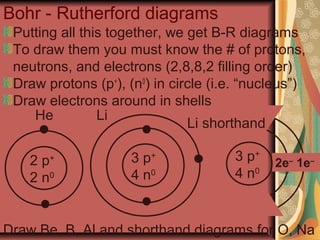
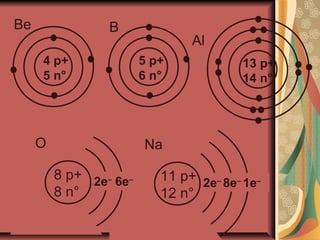
3) At the turn of the 20th century, experiments by Thomson, Rutherford and Bohr led to models of atoms with electrons orbiting a dense positively charged nucleus, accounting for most of an atom's mass. This explained experimental observations.