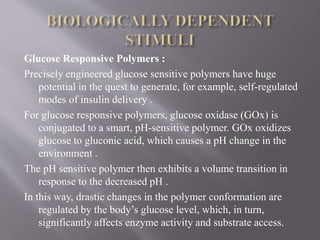
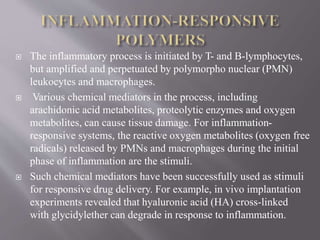
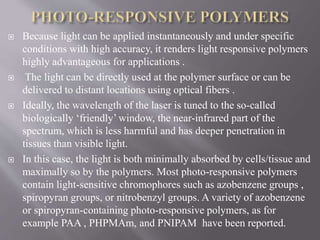
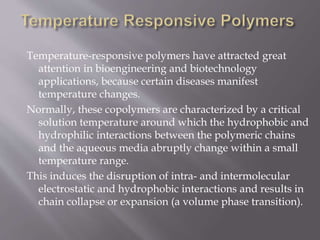
The document discusses various types of stimuli-responsive polymers categorized as physical, chemical, or biological dependent stimuli. It provides details on light, temperature, pH, ion, glucose, enzyme, and inflammation responsive polymers. It notes the advantages of light responsive polymers for biomedical applications and summarizes temperature responsive polymers. Finally, it discusses the potential of dual stimuli polymers that can respond to more than one stimulus simultaneously for improved drug therapies and biomedical applications.


![ Upon ionization, the electrostatic repulsions of the generated
charges (anions or cations) cause a dramatic extension of
coiled chains. The ionization of the pendant acidic or basic
groups on polyelectrolytes can be partial, due to the
electrostatic effect from other adjacent ionized groups.
Another typical pH responsive polymer exhibits
protonation/deprotonation events by distributing the charge
over the ionisable groups of the molecule, such as carboxyl
or amino groups .
pH induces a phase transition in pH responsive polymers
very abruptly. Usually, the phase switches within 0.2–0.3 U
of pH. pH responsive polymers typically include chitosan ,
albumin , gelatin , poly(acrylic acid) (PAAc)/chitosan IPN,
poly(methacrylic acid-g-ethylene glycol) [P(MAA-g-EG)],
poly(ethylene imine) (PEI) , poly(N,N-diakylamino
ethylmethacrylates) (PDAAEMA), and poly(lysine) (PL) .](https://image.slidesharecdn.com/stimuliresponsivepolymerppt1-230918144502-3a37b386/85/Stimuli-responsive-polymer-ppt-1-pptx-7-320.jpg)