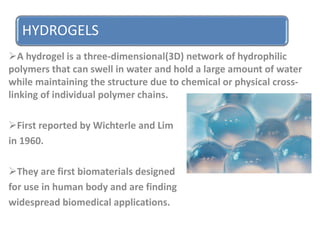
Hydrogels are three-dimensional networks of hydrophilic polymers that can swell in water, first reported in 1960, and are increasingly used in biomedical applications. They can retain large amounts of water due to their chemical or physical cross-linking and are classified based on preparation and cross-linking methods. While hydrogels have advantages such as biocompatibility and flexibility, they also face disadvantages like high cost, low mechanical strength, and challenges in handling and sterilization.