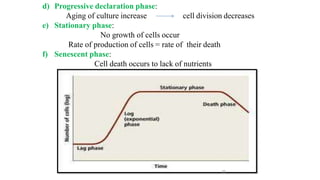
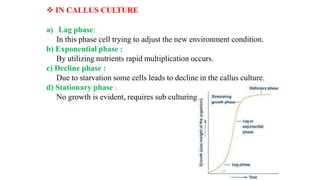
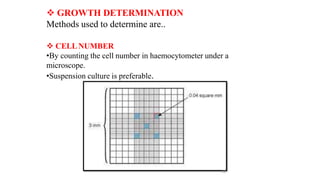
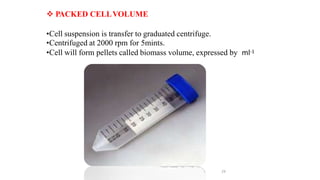
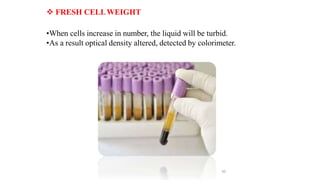
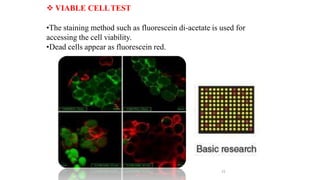
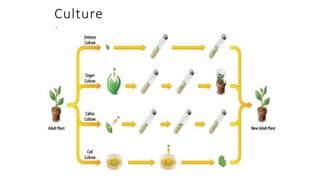
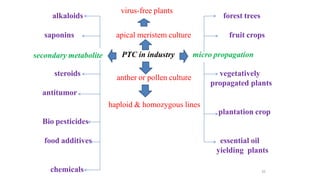
The document describes the steps involved in micropropagation:
1. Explant selection from a donor plant
2. Establishment of the explant in culture media
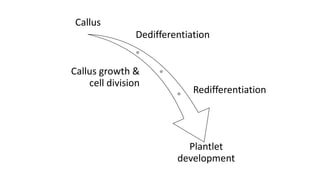
3. Callus development and cell division from the explant
4. Development of plantlets from the callus tissue
5. Hardening or acclimatization of the plantlets for transplanting.