Embed presentation
Download to read offline
























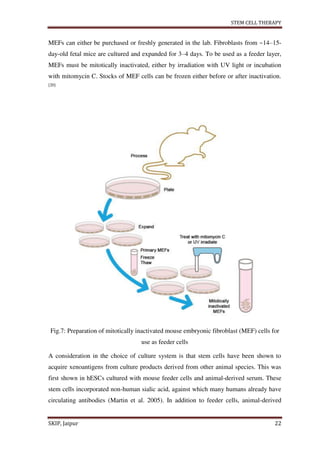













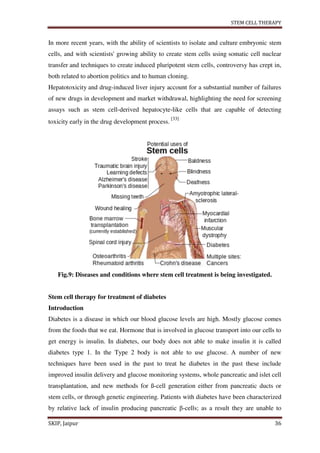













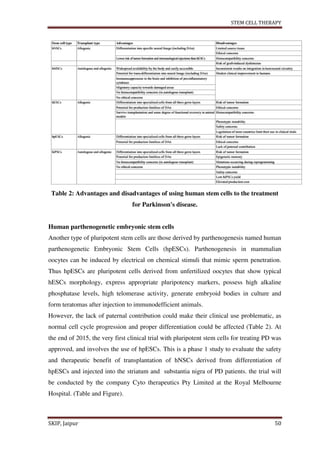






















This document provides an overview of a student project report on stem cell therapy. It includes sections on the introduction, advantages, disadvantages, types of stem cells, sources, isolation and culture, stem cell division, treatment, medical uses, applications to various diseases and conditions. The report was submitted by a pharmacy student, Rishabh Tiwari, to Rajasthan University of Health Sciences, Jaipur, India under the supervision of a faculty member, to fulfill the requirements of a Bachelor of Pharmacy degree.














































































