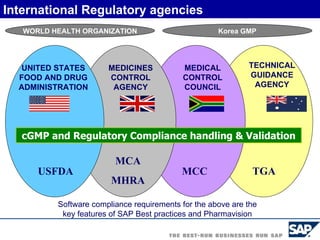
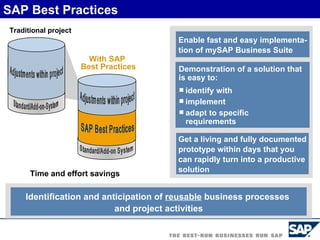
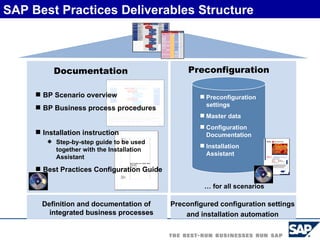
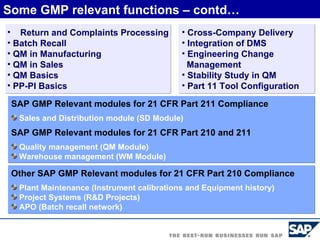
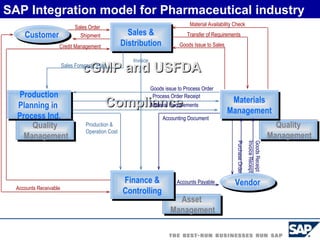
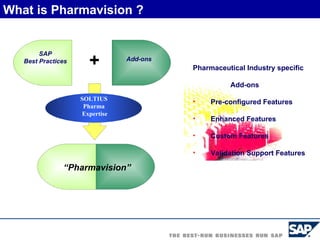
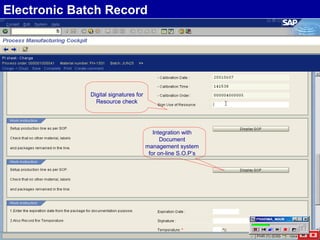
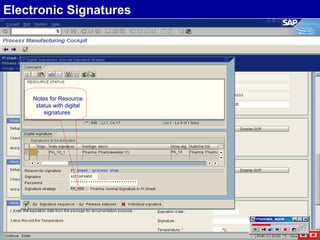
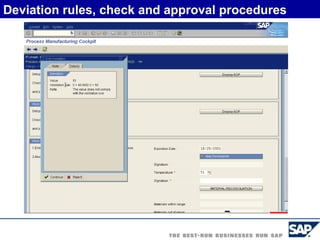
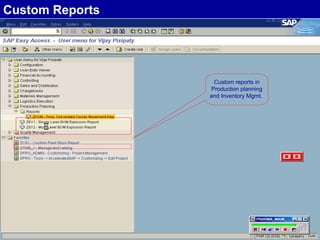
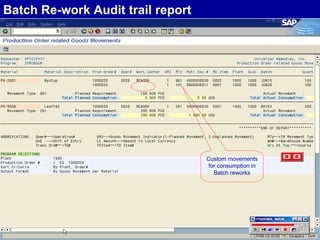
The document discusses the global and Korean pharmaceutical industries and regulations. It provides an overview of SAP Best Practices solutions and Pharmavision add-ons for compliance with FDA 21 CFR Part 11 regulations. Case studies are presented of pharmaceutical companies that successfully implemented SAP Best Practices and Pharmavision.




















![More Information: Contact [email_address] Questions?](https://image.slidesharecdn.com/090618seminarpresentationv1-12452998314-phpapp01/85/SAP-in-Pharmaceutical-Industry-36-320.jpg)