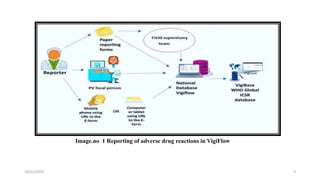
Vigiflow is a web-based system for managing individual case safety reports (ICSRs) related to adverse drug reactions and events. It allows for complete data entry on reported cases and integrated dictionaries to help with correct coding. Vigiflow provides easy connection between regional and national servers to share reported case data. While it offers free-text fields and constant data sharing, some disadvantages include potential server issues between countries and need for fast internet connections. Vigiflow has been used since 2001 and allows authorities and companies to manage safety report databases and conduct data analysis.