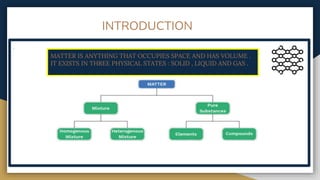
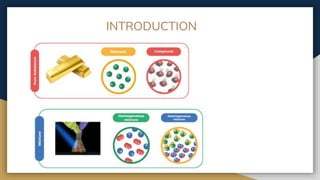
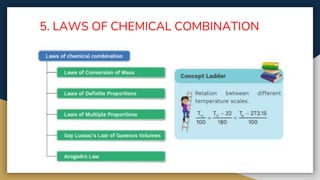
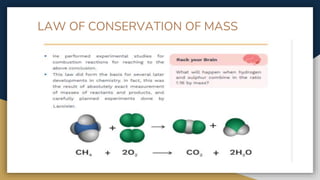
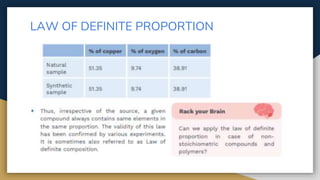
The document outlines the syllabus for 11th grade chemistry for the 2024-2025 session, covering fundamental topics such as stoichiometry, the mole concept, and states of matter. It emphasizes key concepts like precision and accuracy, laws of chemical combination, and concentration terms. The content serves as an introduction to essential chemical principles and practical applications.





















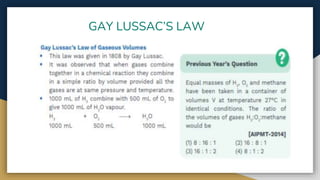



![Mole Concept
03
..
SUBTOPICS
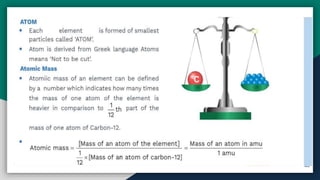
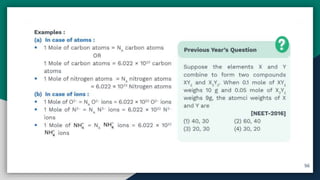
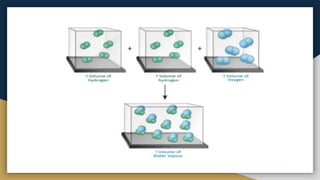
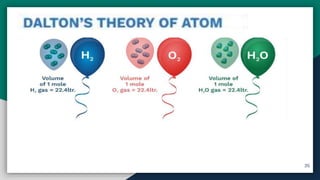
➊ Atom , Atomic Mass [ amu/u ] , Molecular mass
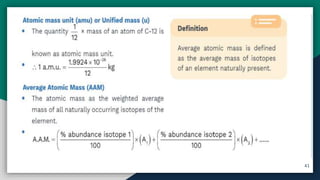
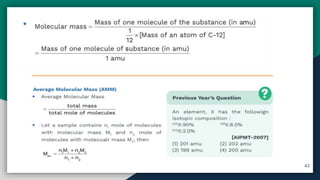
➋ Average Atomic Mass & Molecular Mass
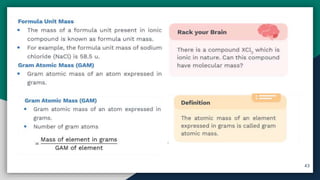
➌ Formula Unit Mass , GAM , GMM
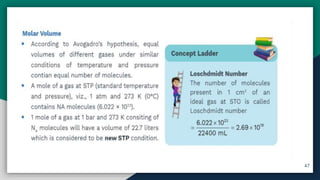
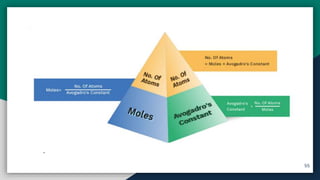
➍ Mole Concept , Molar Mass & Molar Volume
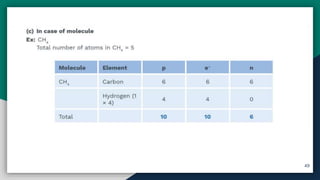
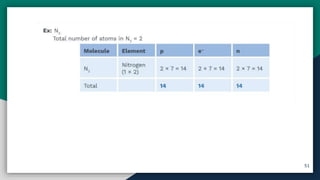
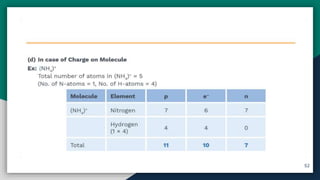
➎ Basic Concepts Of Atom
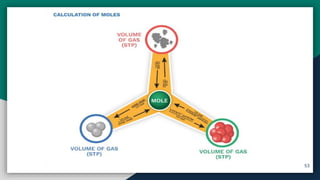
➏ Calculation of Moles
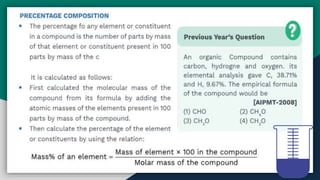
➐ Percentage Composition
➑ Empirical Formula and Molecular Formula](https://image.slidesharecdn.com/91bl2owrx2wqtrrbdzl8-science-240608135752-b46e31bf/85/SOME-BASIC-CONCEPTS-OF-CHEMISTRY-CLASS11-38-320.jpg)