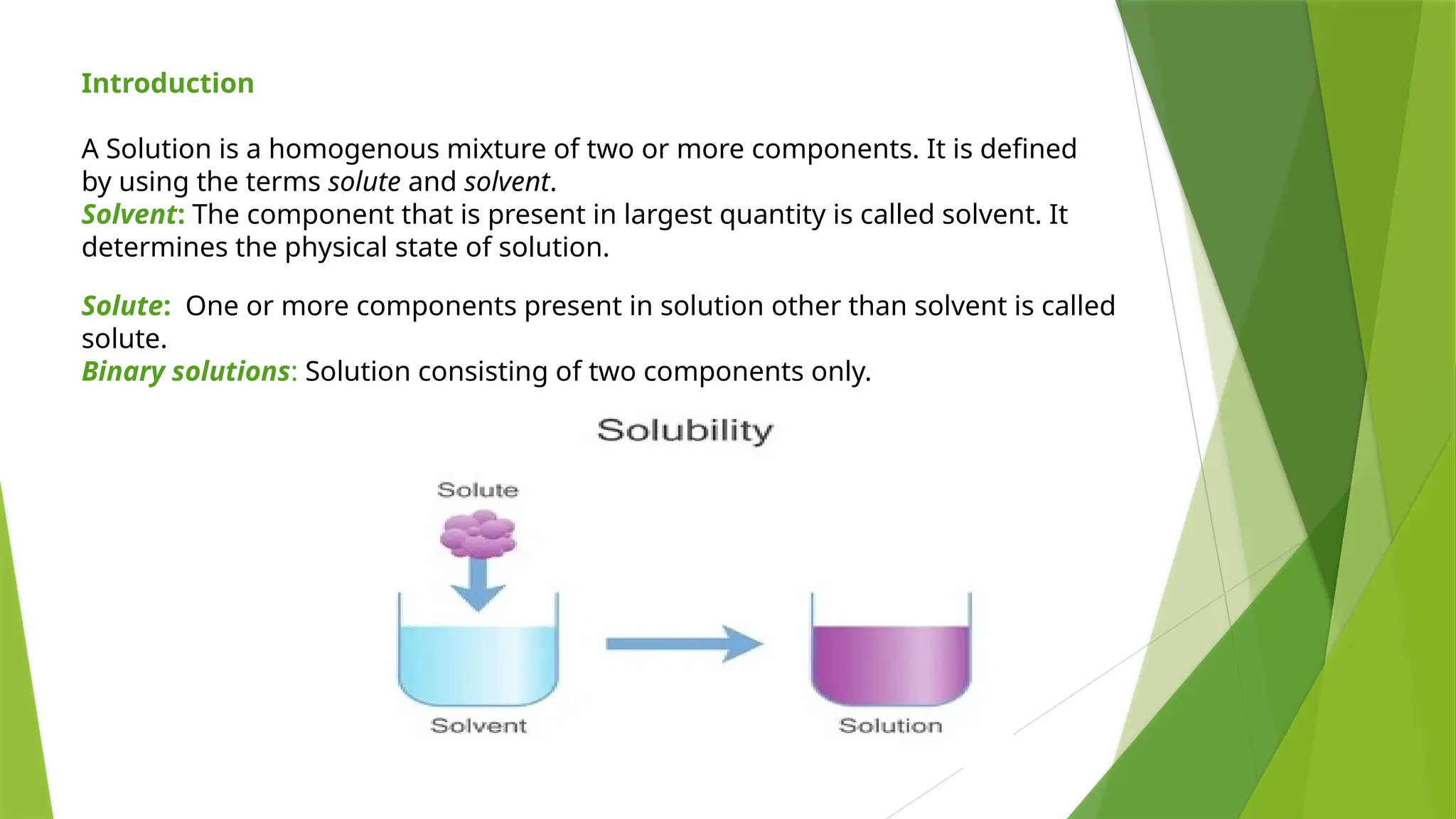
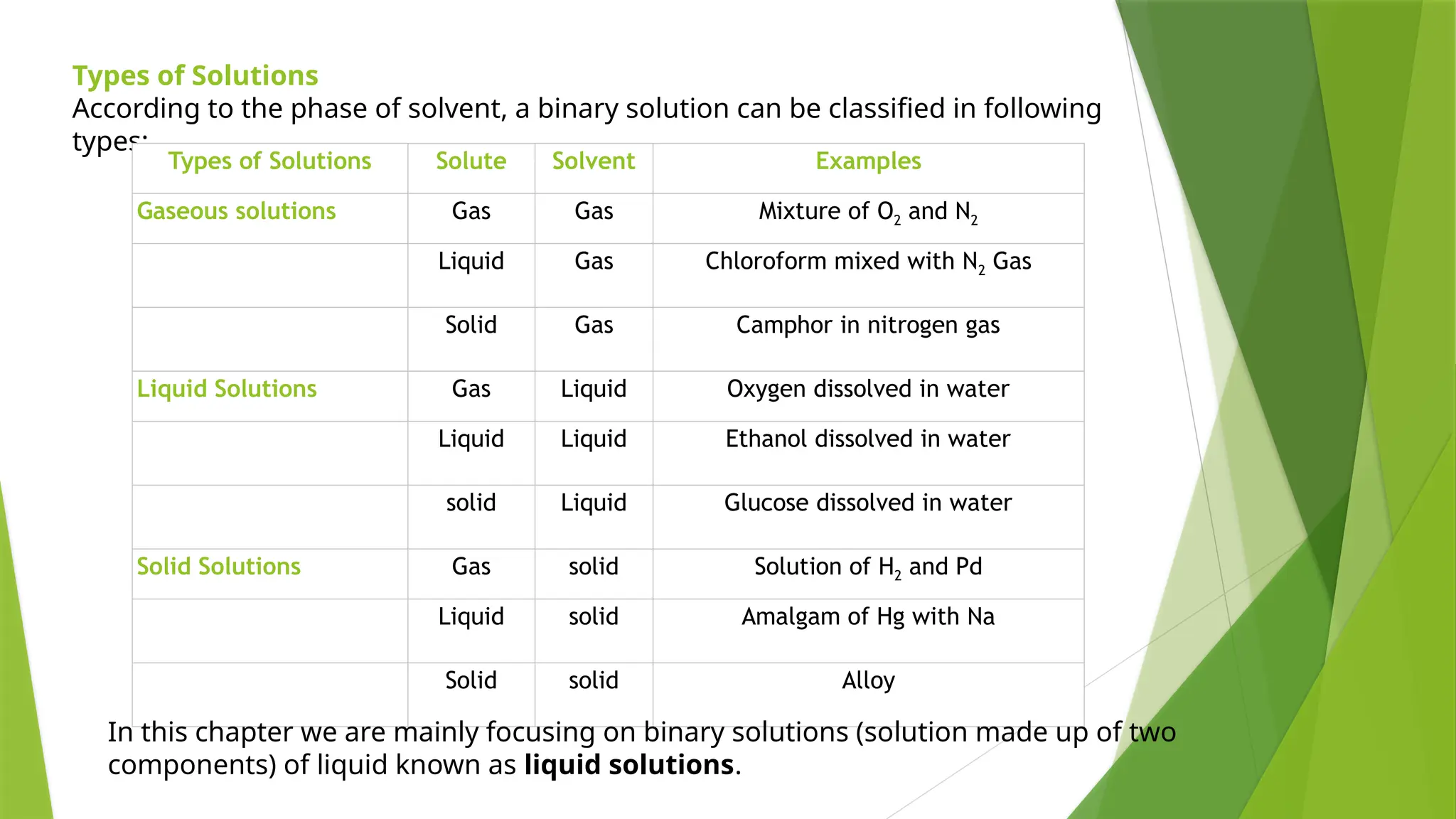
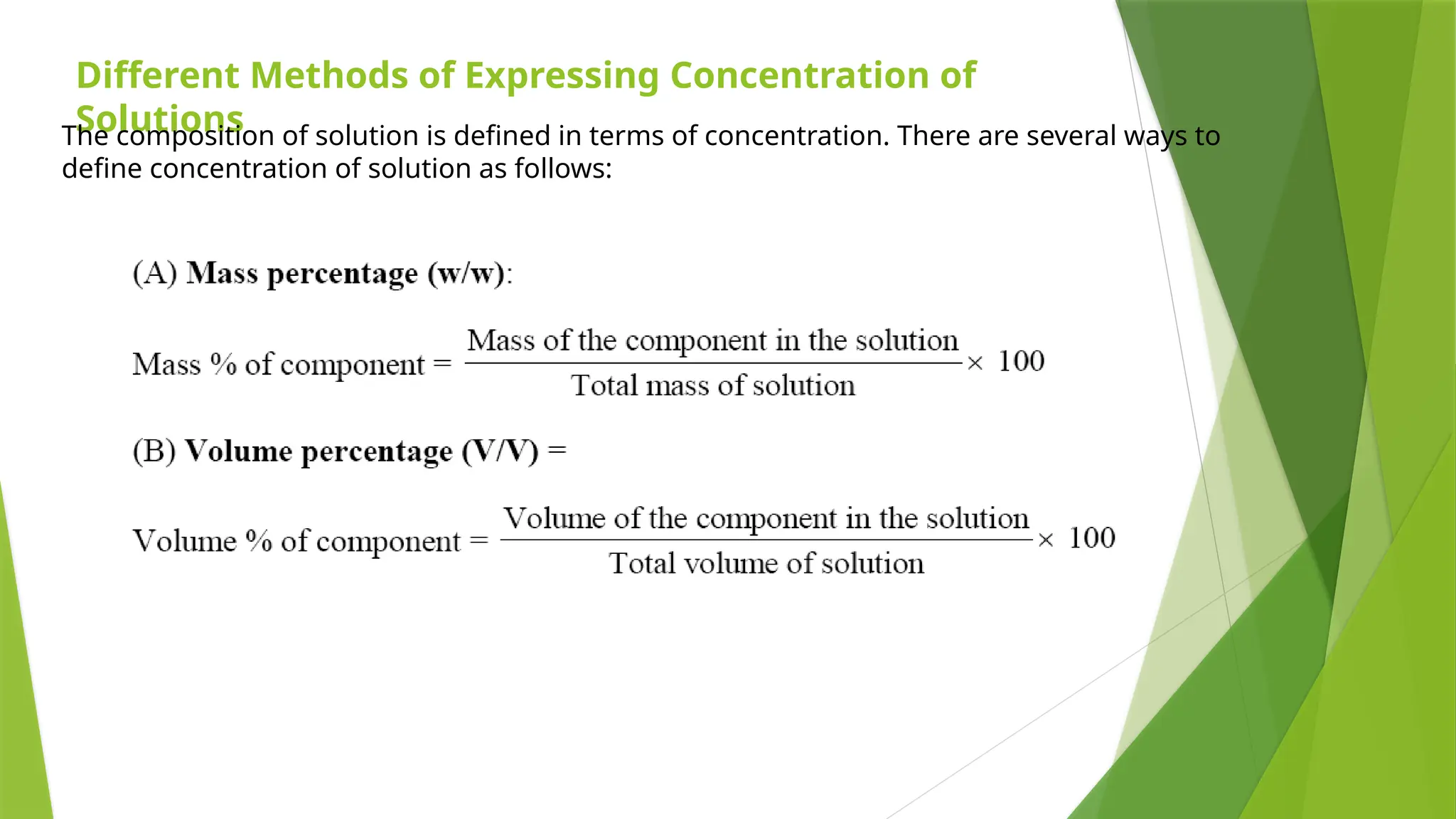
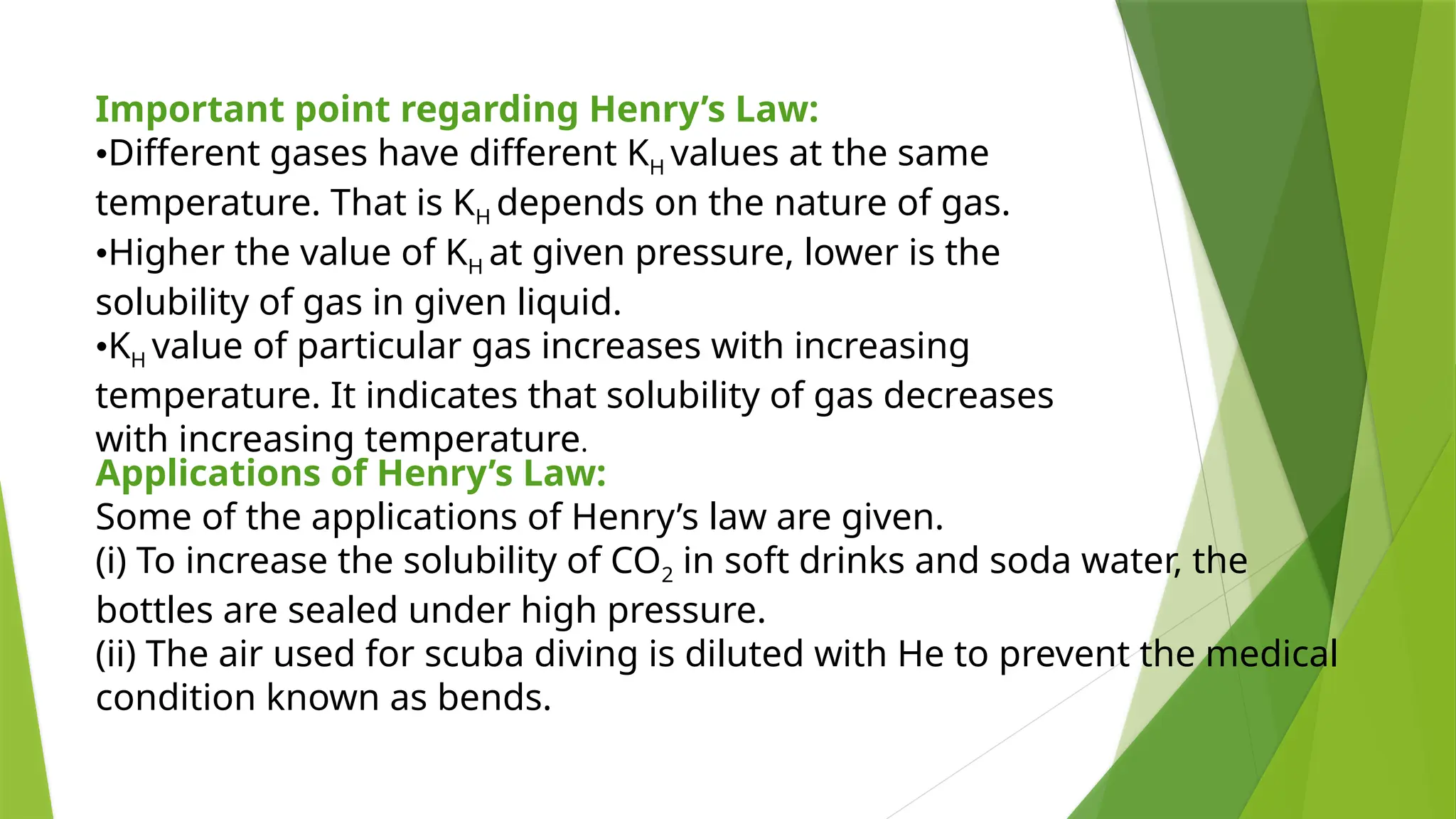
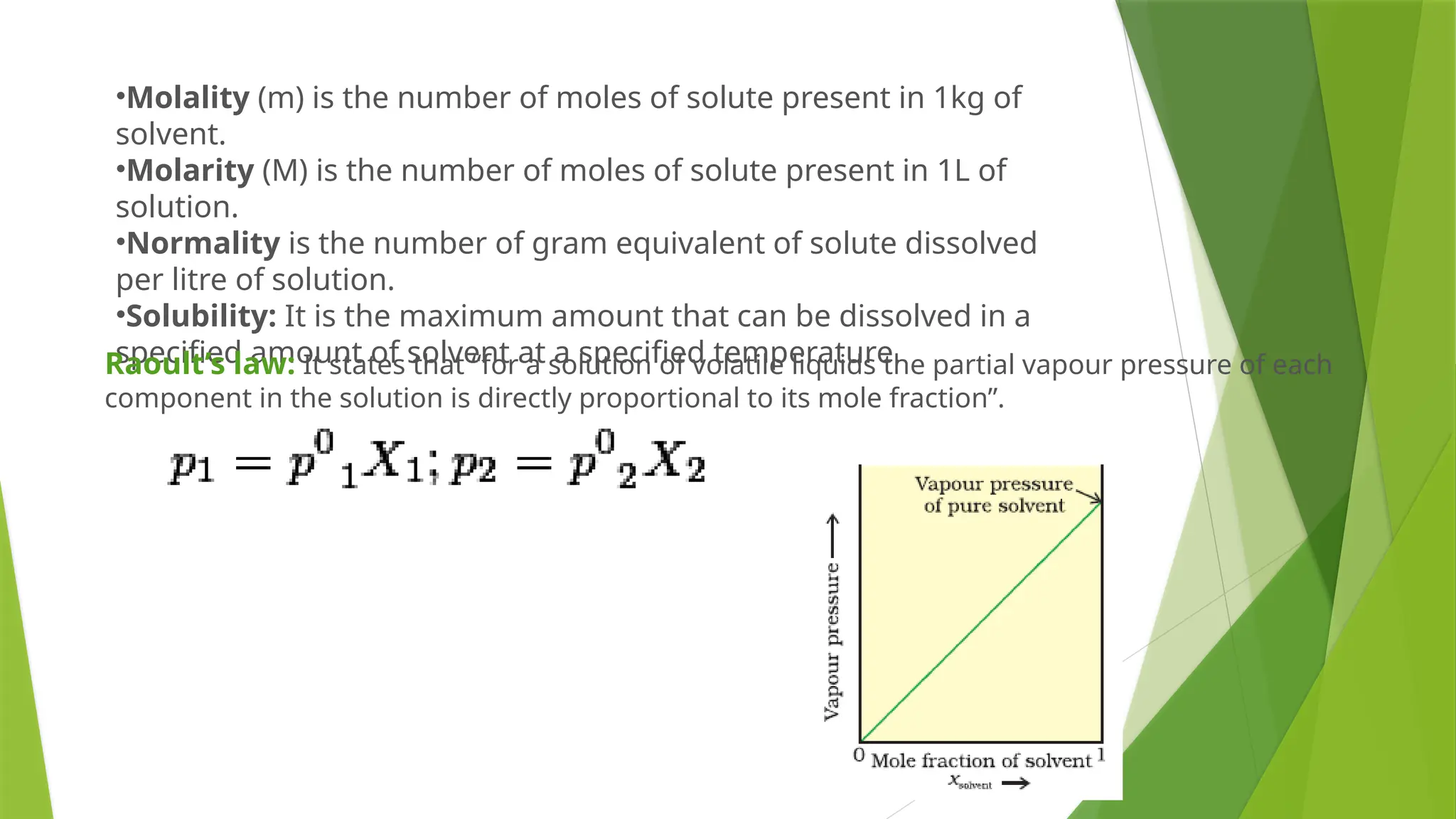
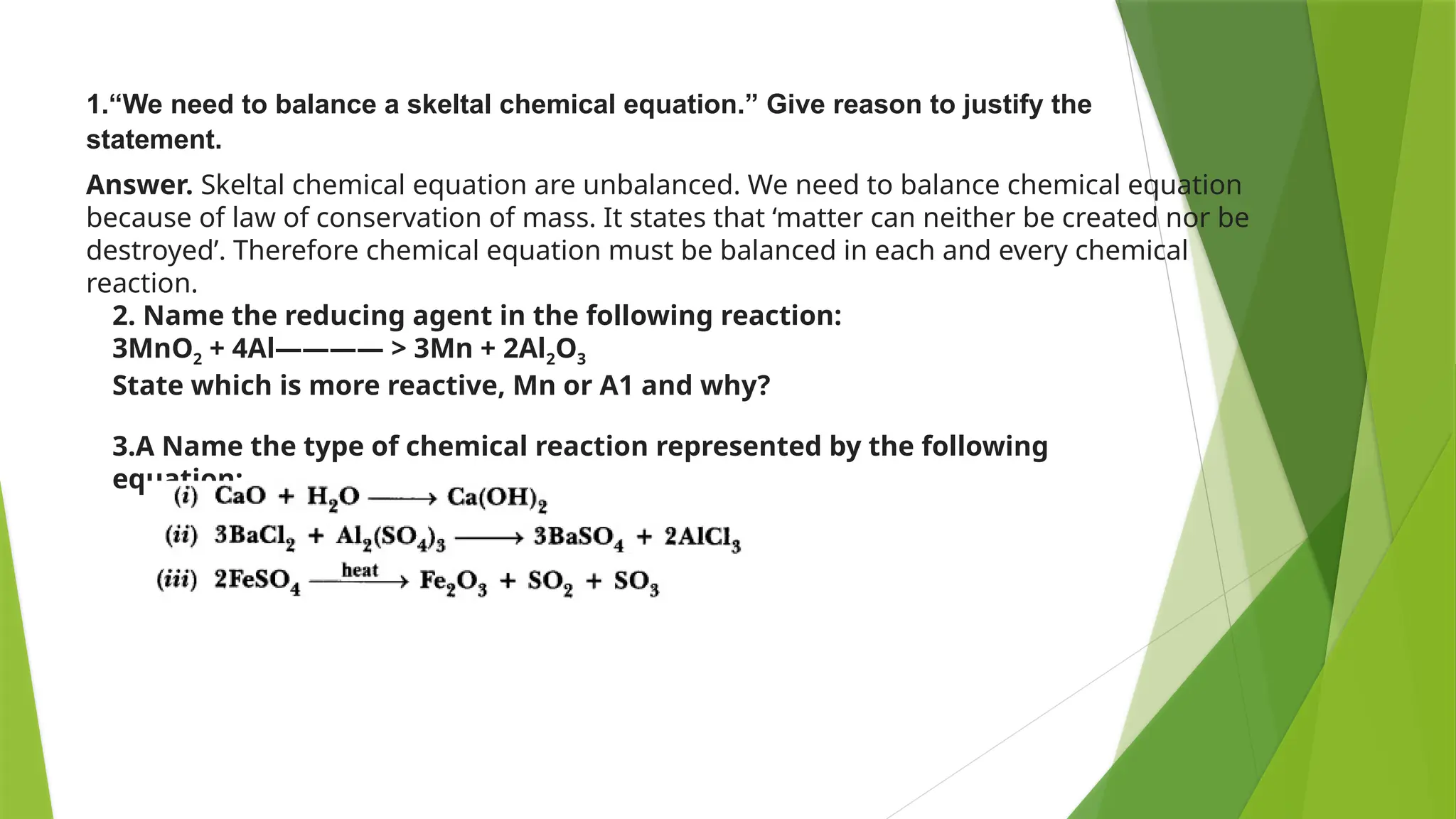
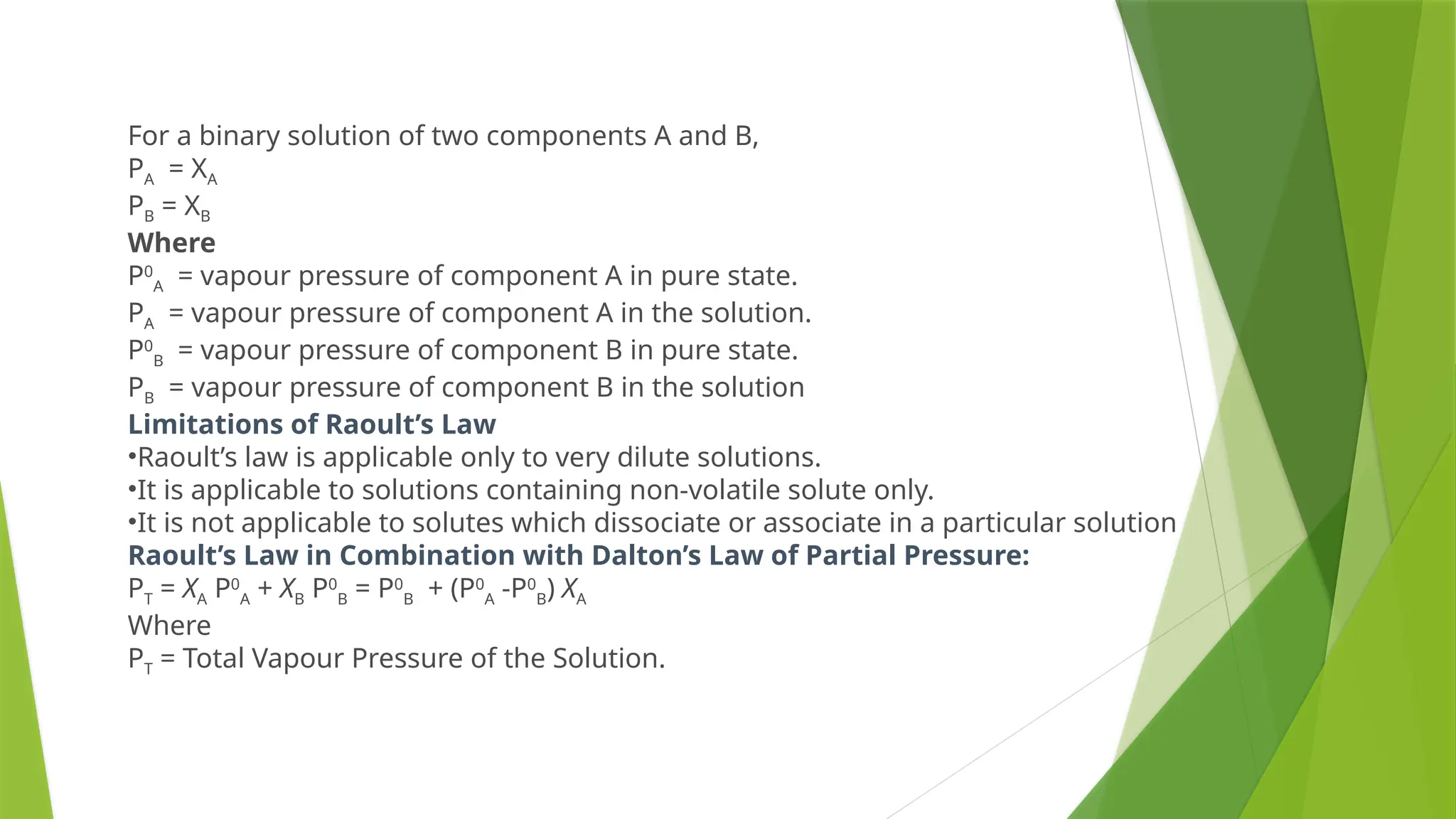
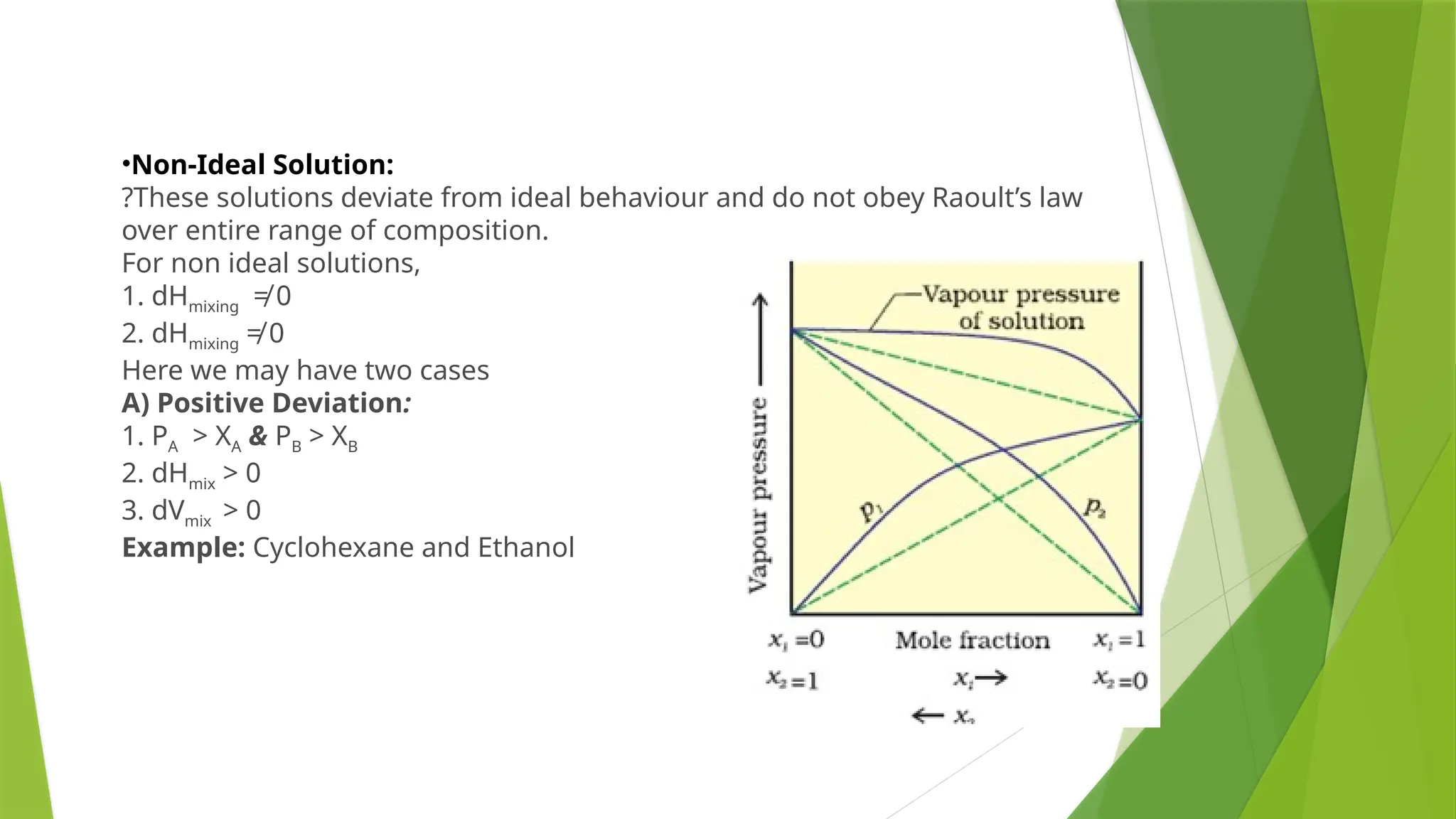
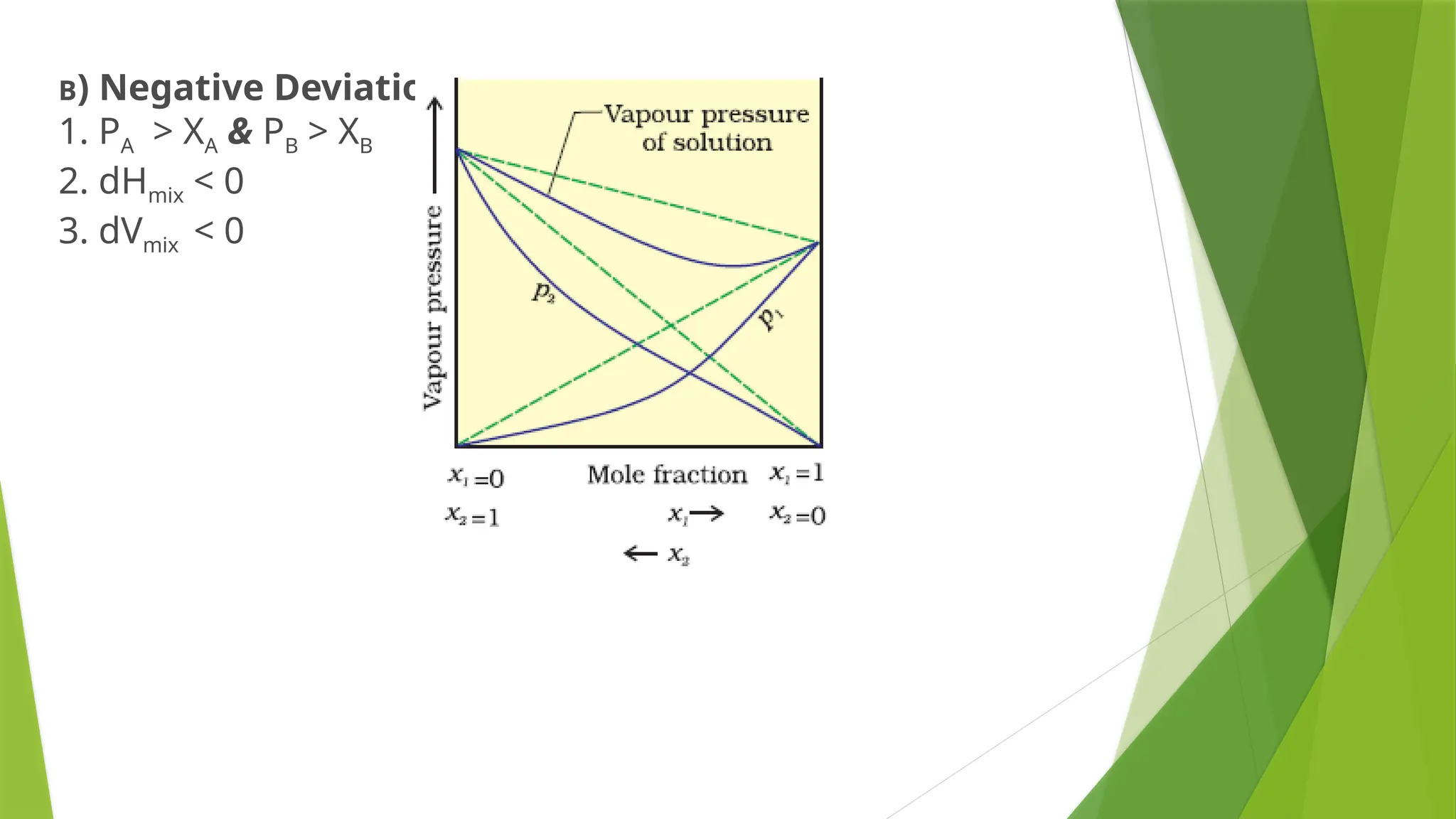
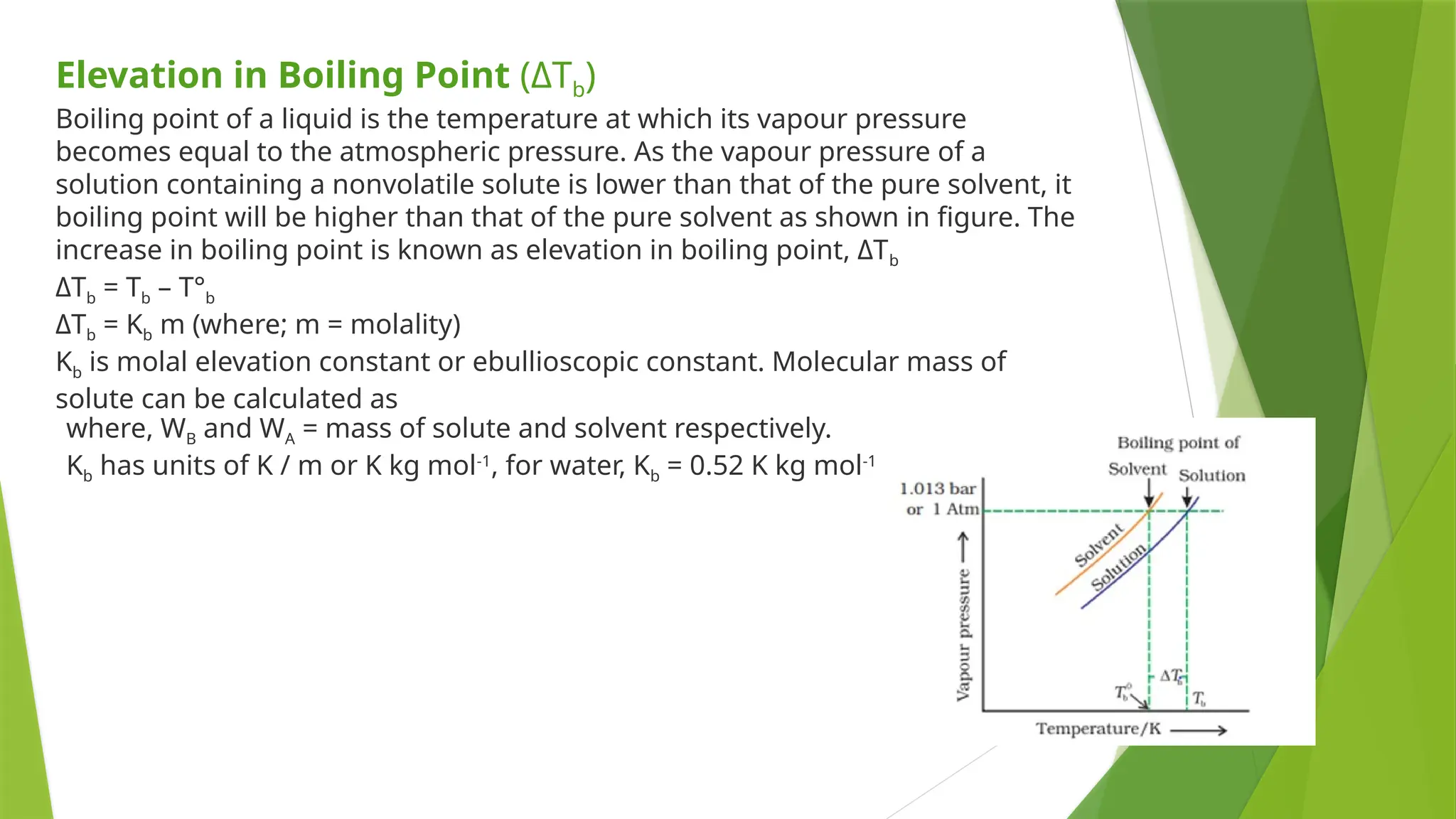
This document provides an overview of solutions, focusing on binary liquid solutions and their classifications. It discusses the factors affecting solubility, methods of expressing concentration, Raoult's law, Henry's law, and concepts related to osmotic pressure. Additionally, it elaborates on ideal and non-ideal solutions and important characteristics like boiling point elevation and osmotic phenomena.


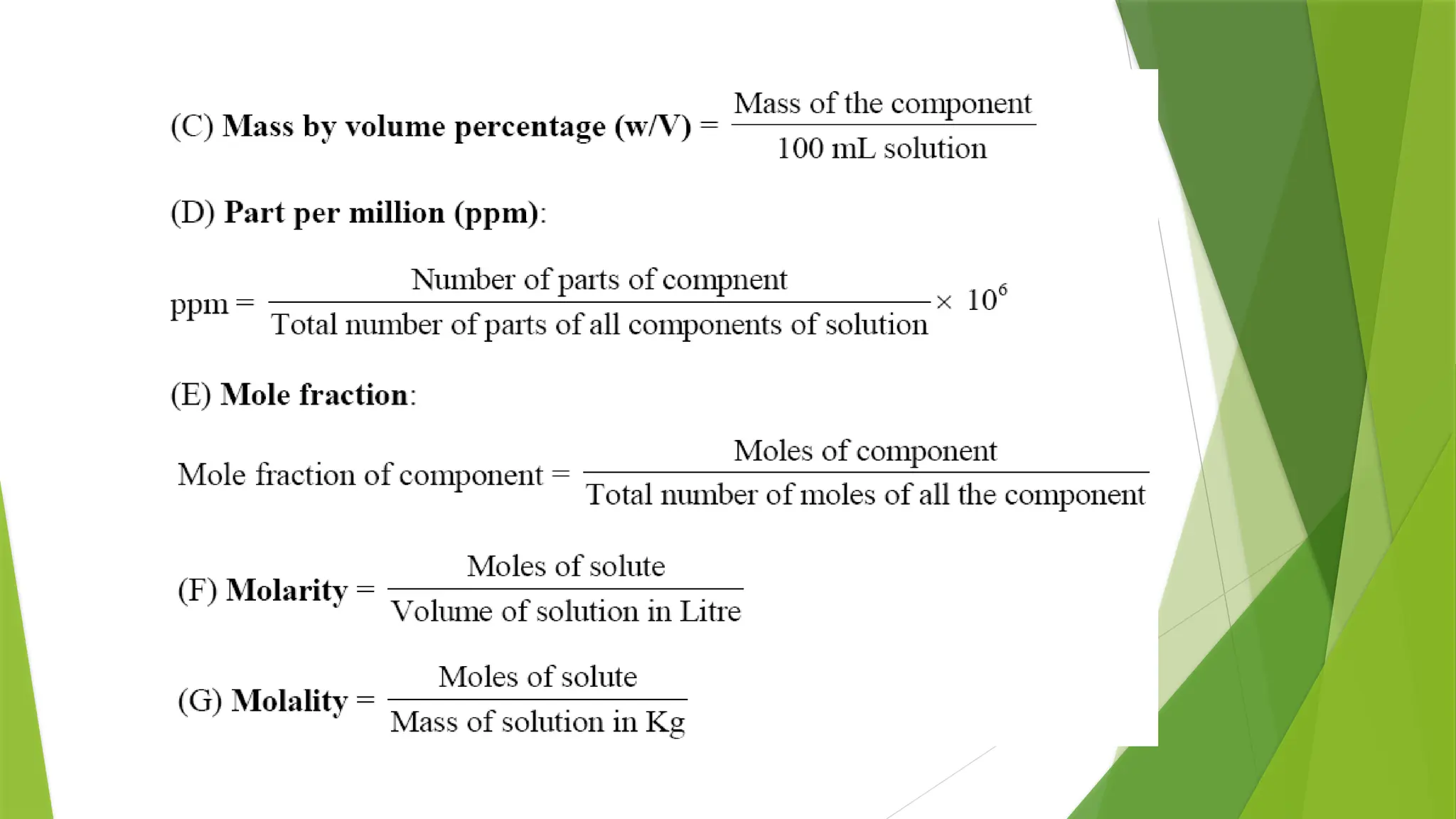
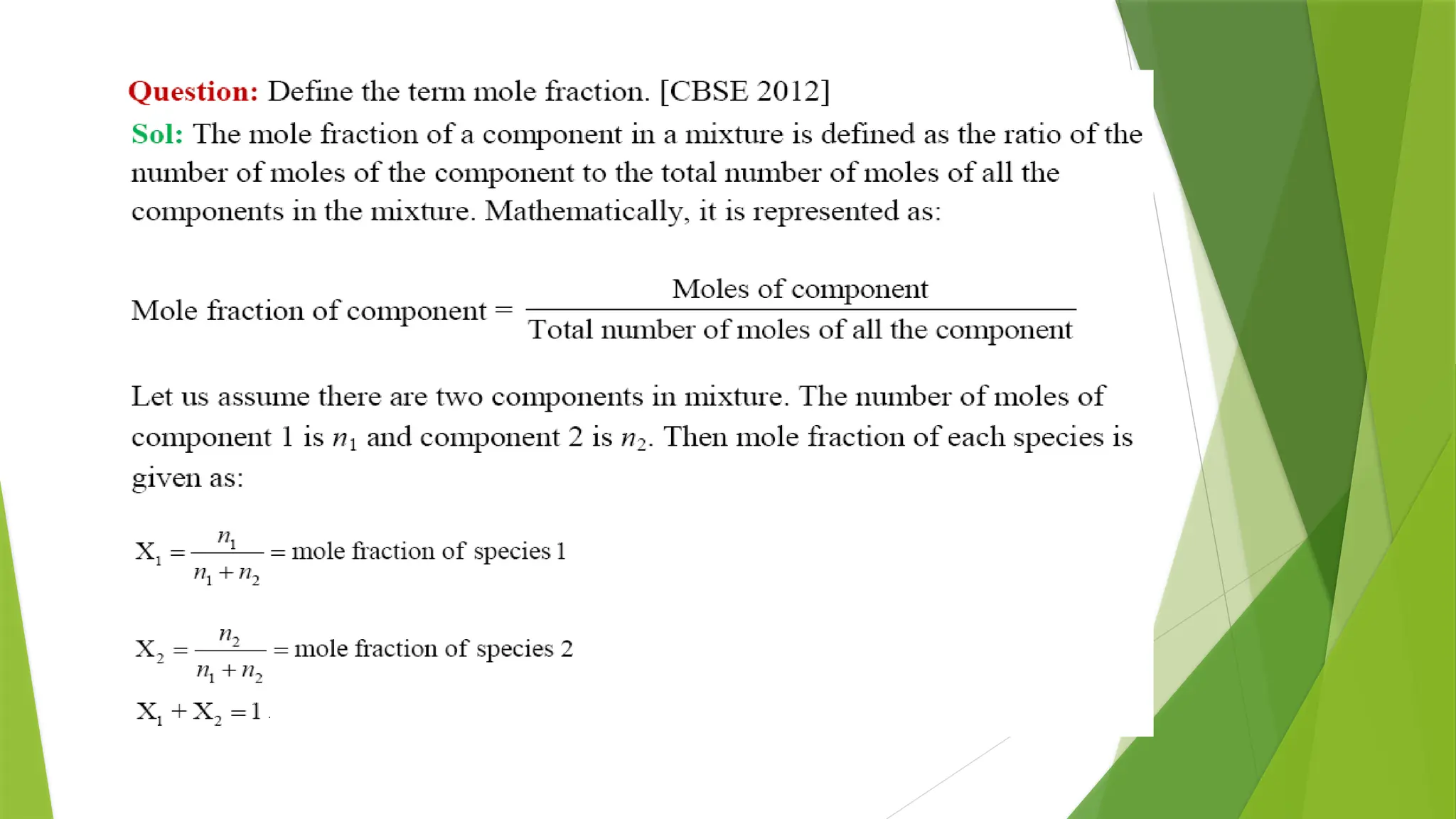

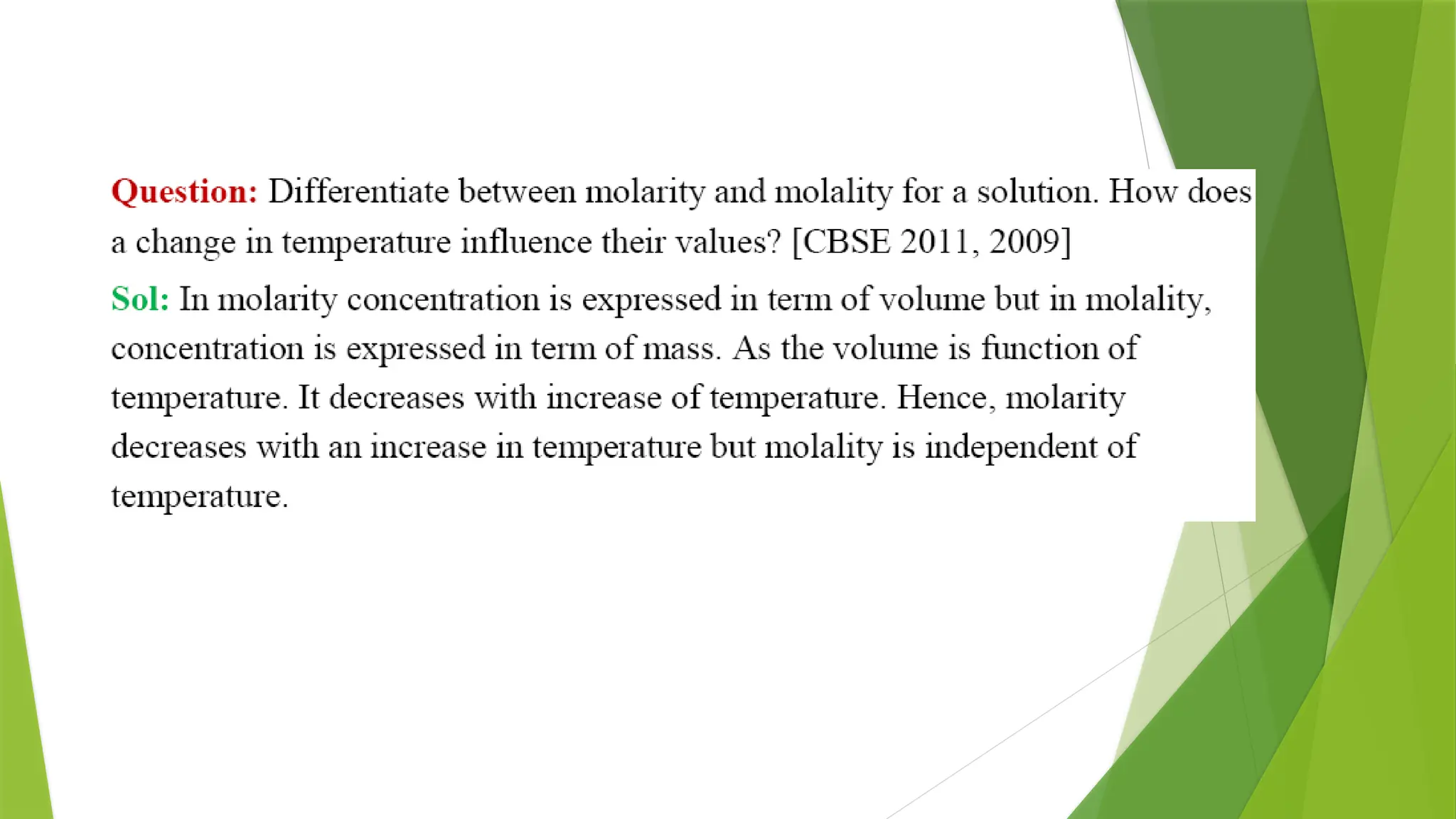







![Osmotic Pressure (π)
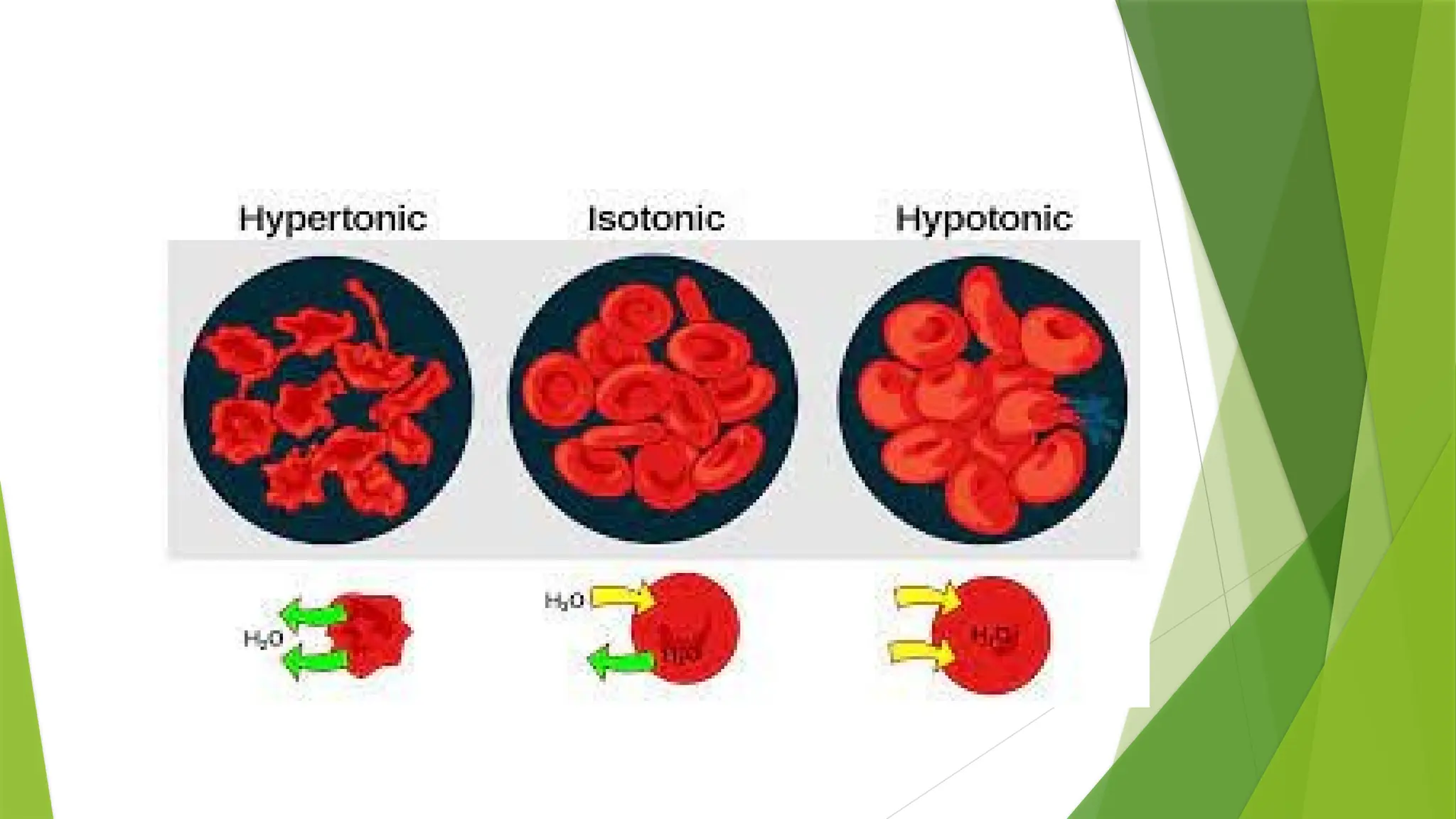
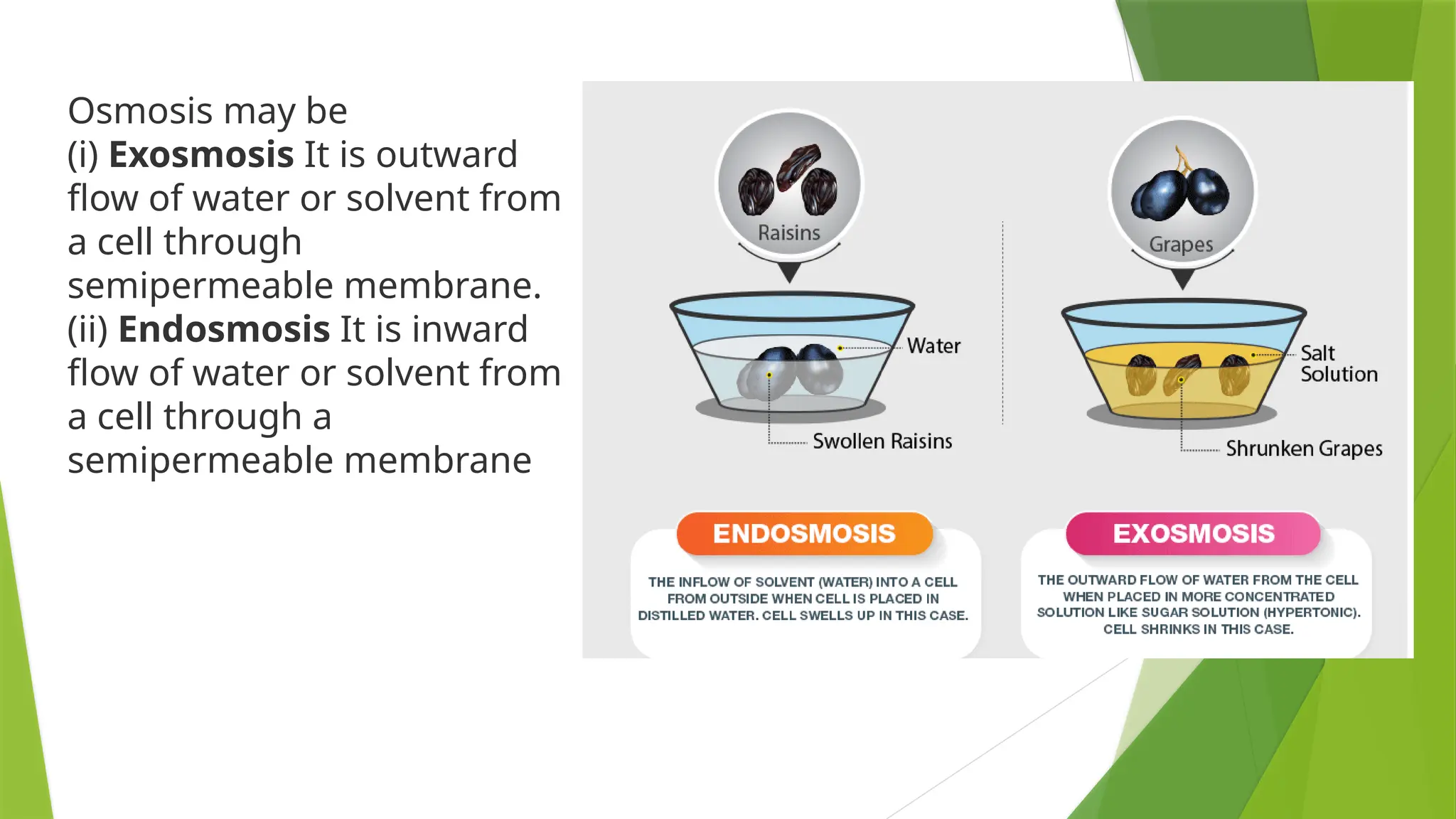
Osmosis is the phenomenon of
spontaneous flow of the solvent
molecules through a
semipermeable membrane from
pure solvent to solution or from a
dilute solution to concentrated
solution. It was first observed by
Abbe Nollet.
Some natural semipermeable
membranes are animal bladder,
cell membrane etc.
CU2[Fe(CN)6],CELLULOSE AND
ACETATE is an artificial
semipermeable membrane which
does not work in non-aqueous
solutions as it dissolves in them.
.](https://image.slidesharecdn.com/solutionclass12-220703150819-2da48a28-240903055739-85f5526d/75/solution-class-12-pptx-chemistry-book-1-chapter-1-25-2048.jpg)
![Osmotic pressure of a solution is equal to the external pressure applied on
higher conc. side so as to just stop the process of osmosis. [External pressure
= ∏ ]](https://image.slidesharecdn.com/solutionclass12-220703150819-2da48a28-240903055739-85f5526d/75/solution-class-12-pptx-chemistry-book-1-chapter-1-27-2048.jpg)