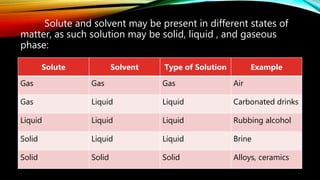
1. A solution is a homogeneous mixture of two or more substances, containing a solute dispersed in a solvent.
2. Solutions have uniform composition throughout and their components can be separated through physical means like evaporation or crystallization.
3. The solubility of a solute is affected by factors like particle size, temperature, pressure, and concentration unit/amount of solute present in the solution. Increasing temperature or decreasing particle size generally increases solubility.