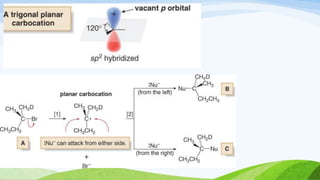
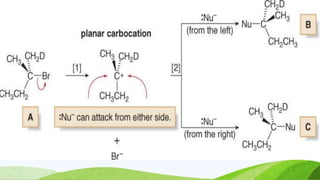
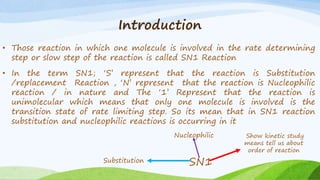
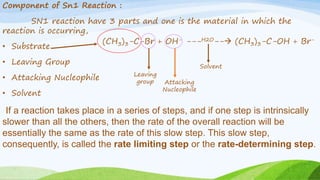
SN1 reaction involves a single molecule in the rate determining step. It is a two-step reaction where the first step is bond breaking to form a carbocation intermediate and the second step is nucleophilic attack. SN1 reactions are favored by stable carbocations, polar protic solvents, and tertiary alkyl halides since these allow for easier formation of the carbocation intermediate. The reaction is first order with respect to the substrate and undergoes racemization when a chiral substrate is used. Nucleophilic attack occurs from the top and bottom sides of the planar carbocation.


![Substrate-leaving group [substrate]
++ [Leaving group]
-
Rate = [substrate ]
*The question that arises here is what motivates it? Are there external
reasons that cause the leaving group to leave easily?
* Why it’s Rate only depend upon the substrate ?
Step 2: Here bond forming is occur between the positive charge substrate
and Attacking Nucleophile. This step is exothermic in nature because here a
bond formation is occurring and we know when bond formation is occurring
then energy is released to the environment and here a lot of energy is
release . This step is the fast step of the reaction because here Ions are
involved which required very low activation energy to combine because they](https://image.slidesharecdn.com/sn1reaction-210701182756/85/Sn1-reaction-6-320.jpg)
![have itself very high energy and unstable and tending to losing energy and
to gain stability.
In this step :
• Nucleophile addition is occurring
• Highly stable product is formed
[Substrate]
+ + Nu
-/Nu
.. Substrate Nu
This step is not the rate determining step because it’s fast step and we
only consider the slowest step is the rate determining step.
Fast
Step](https://image.slidesharecdn.com/sn1reaction-210701182756/85/Sn1-reaction-7-320.jpg)



![reactant is less than its takes greater time to reach the completion and
similarly, if the rate of a reaction does not depend upon the concentration
of reactant then the time during which the reaction is completing does not
increase or decrease with decrease or increase of concentration of that
reactant, Keeping this concept in mind we have to proof that SN1 reaction
only depend upon concentration of Substrate by a practical work .
Practical work :
Let suppose ! We have a reaction ,
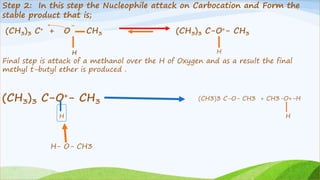
(CH3 ) 3 C – Br + CH3- O H (CH3 ) 3 C – O–CH3 + HBr
The rate equation for the above reaction is;
Rate = [ (CH3)3C- Br] [CH3-OH]
Methanol
boil
*Why it’s Rate only depend
upon the substrate ?](https://image.slidesharecdn.com/sn1reaction-210701182756/85/Sn1-reaction-12-320.jpg)
![Experiments:
From the above experiment it is clear that rate does not change with change
in concentration of CH3-OH( Nucleophile).
So its proof that rate is only depends upon concentration of Substrate that
is (CH3)3 C- Br.
S.N
O
[(CH3)3C-
Br]
[CH3 –
OH]
Time of
completion of
reaction
Rate of reaction
01 0.01ml 0.01 5 seconds X
02 0.02ml 0.01ml 2.5 seconds 2x
03 0.01ml 0.02ml 5 seconds x
04 0.02ml 0.02ml 2.5 seconds 2x](https://image.slidesharecdn.com/sn1reaction-210701182756/85/Sn1-reaction-13-320.jpg)