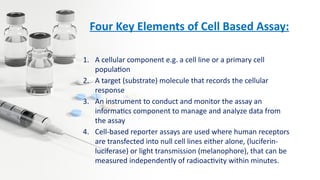
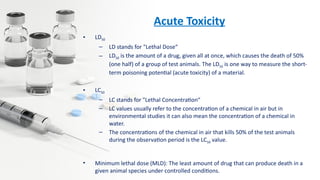
Pre-clinical drug development involves several key stages: high throughput screening to identify potential drug candidates, toxicology studies in animal models to determine safety, pharmacological profiling to understand mechanisms of action, and calculating initial human doses. The overall goals are to obtain sufficient data on safety, tolerability and efficacy to receive regulatory approval from the FDA to begin clinical trials in humans. Pre-clinical studies provide critical data required for an Investigational New Drug (IND) application to the FDA.












































![Repeated dose toxicity studies
(Sub acute/ sub chronic toxicity)
• Aim:
– To identify Target Organ Toxicity
– To establish MTD for subsequent studies
• Animal- at least 2 mammalian species(1 rodent & 1 non- rodent)
– Rodent- must be between 6-8 weeks
– Non-rodent should be between 4-9 months
– Younger and still growing animals are preferred in initiation of sub-chronic
studies
• Drug is given on 14, 28, 90 & 180 days.
• 3 other groups are formed :
– Highest dose Observable toxicity [MTD]
– Lowest dose No observable toxicity [NOAEL] should be comparable to intended
therapeutic dose or multiple of it
– Intermediate dose Some symptoms ; not gross toxicity or death placed
logarithmically between two doses](https://image.slidesharecdn.com/preclinicaldevelopmentfinal-240304022603-884d4d67/85/PreClinical-Development_Final_clinical-pdf-47-320.jpg)












