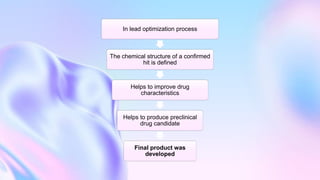
The document outlines the drug development process, which includes steps such as target selection, lead identification, lead optimization, and clinical testing. It discusses various methods and phases involved in drug discovery, testing, and characterization, emphasizing the importance of pre-clinical and toxicological assessments. Additionally, it highlights the need for investigational new drug applications (IND) to ensure safety in human trials.