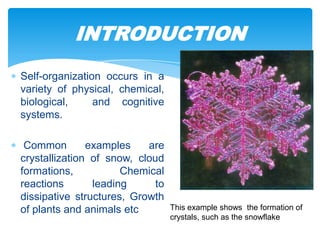
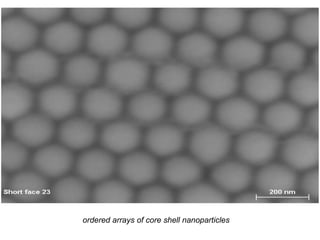
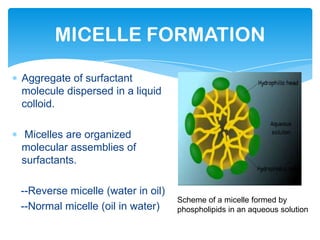
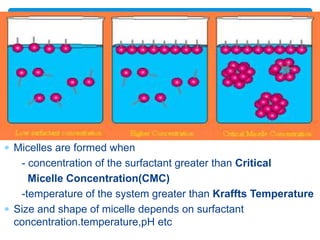
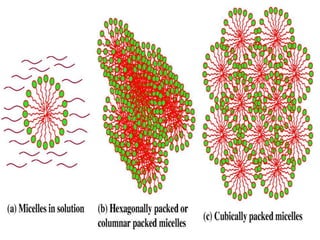
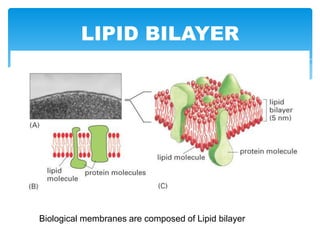
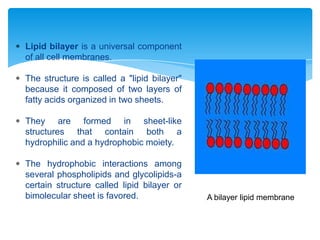
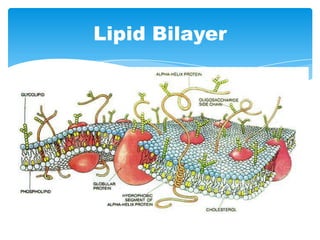
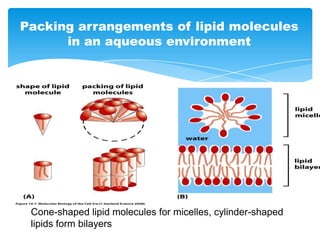
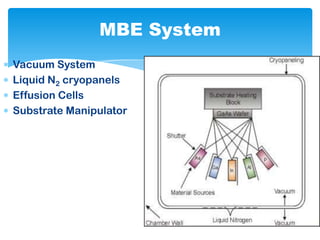
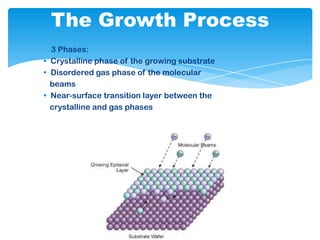
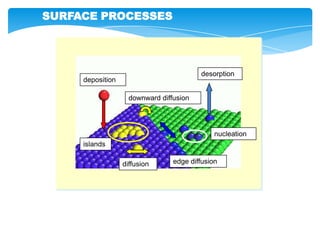
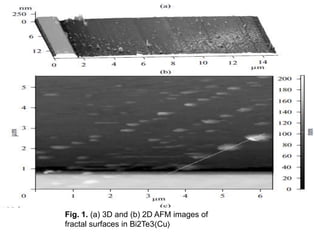
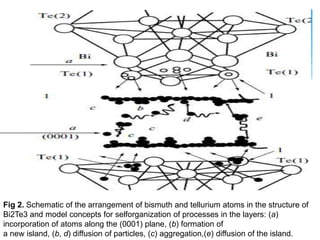
The document discusses self-organization of nanostructured materials. It begins with introducing self-organization as a spontaneous process where ordered structures can arise from local interactions between initially disordered components without external direction. It then describes different kinds of self-organization like micelle formation, lipid bilayers, and molecular beam epitaxy. Specific examples of self-organized nanostructures include ordered arrays of core-shell nanoparticles and nanostructures formed on interlayer surfaces of layered crystals. In conclusion, the document emphasizes that self-organization is an efficient method for constructing nanostructured materials and an area of significant research interest across many fields.