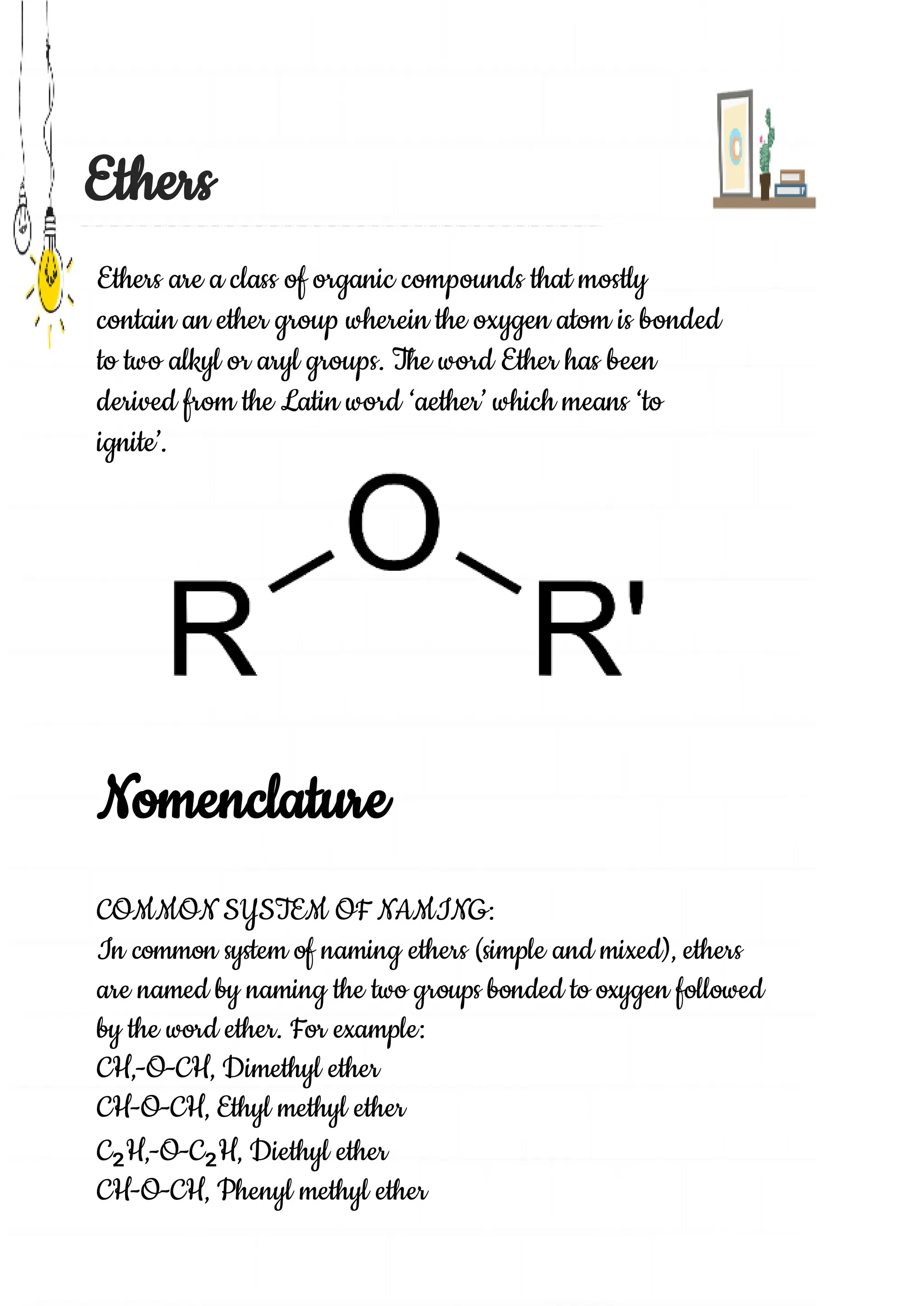
Uttam, a 12th grade student, completed a chemistry project on ethers under the guidance of his teacher Mr. Bineet Kumar Pathak. The project report discusses the topics of nomenclature, preparation, physical and chemical properties of ethers. It explains common and IUPAC systems of naming ethers and methods of preparation such as Williamson's synthesis and reaction of alkyl halides with dry silver oxide. The report also describes the physical properties of ethers such as low boiling points and ability to dissolve organic compounds. It discusses chemical reactivity including cleavage by acids like HI and HBr to form alcohols and alkyl halides.