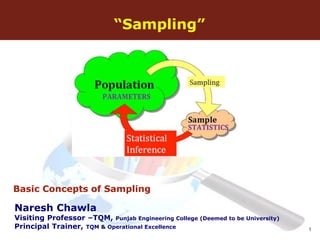
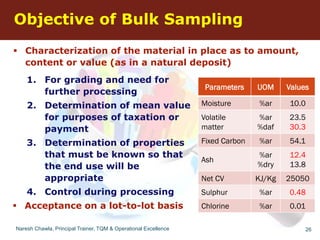
The document discusses the fundamental concepts of sampling, including its purpose, methods, and errors associated with sampling in various fields such as social sciences and manufacturing. It highlights the importance of representative samples, appropriate sample sizes, and acceptance sampling standards, particularly in relation to quality control and regulatory compliance. Historical context, applications, and standards for acceptance sampling are also presented, emphasizing the significance of sampling in ensuring quality across different industries.