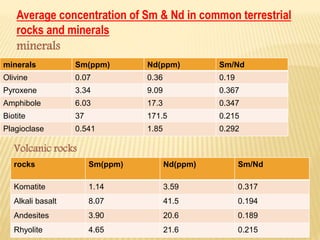
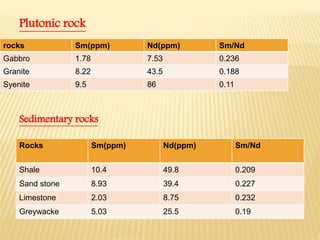
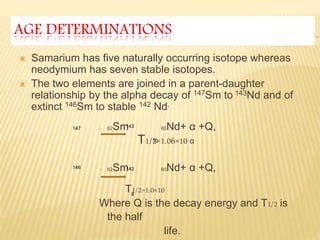
This document discusses the radioactive decay of samarium-147 to neodymium-143 and its use in radiometric dating of rocks and meteorites. It provides background on samarium and neodymium as rare earth elements, their concentrations in common minerals and rocks, and how their ratio changes over time. The document also describes how the samarium-neodymium dating method is used to determine the age of the Earth based on analysis of meteorites and how it can be applied to date terrestrial and lunar rocks.