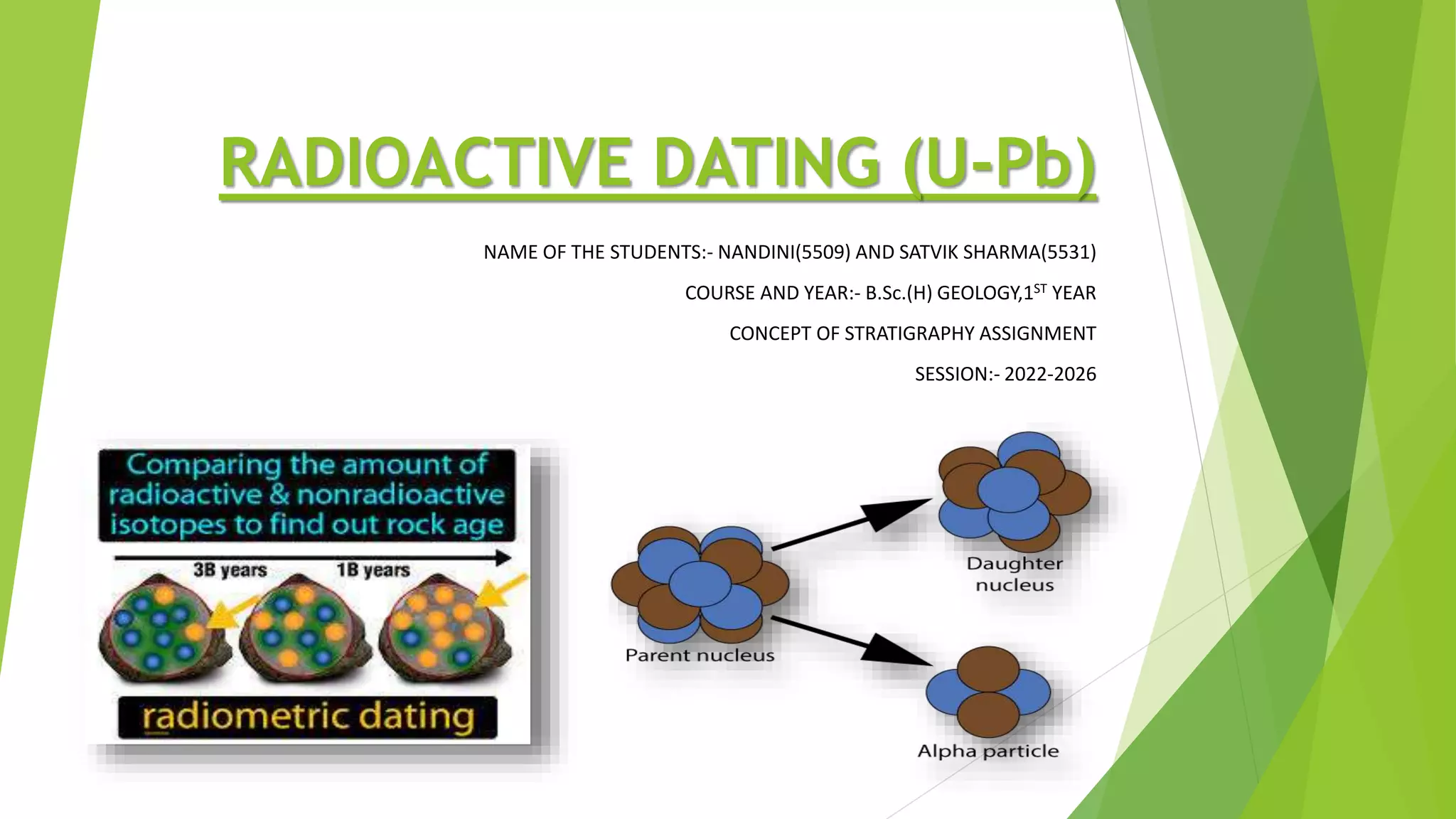
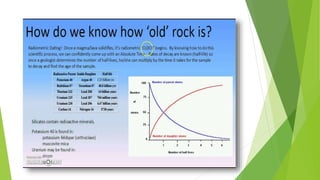
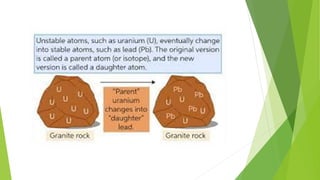
Geologists use radiometric dating techniques like uranium-lead dating to determine the age of rocks and fossils. Uranium-lead dating relies on the radioactive decay of uranium isotopes U-238 and U-235 into lead isotopes over millions to billions of years. The technique can date materials from 1 million to over 4.5 billion years old with high precision, but works best with minerals like zircon that fully retain the uranium and lead. While very accurate when applied properly, uranium-lead dating of zircon can sometimes produce confusing results requiring special treatment of the zircon samples.