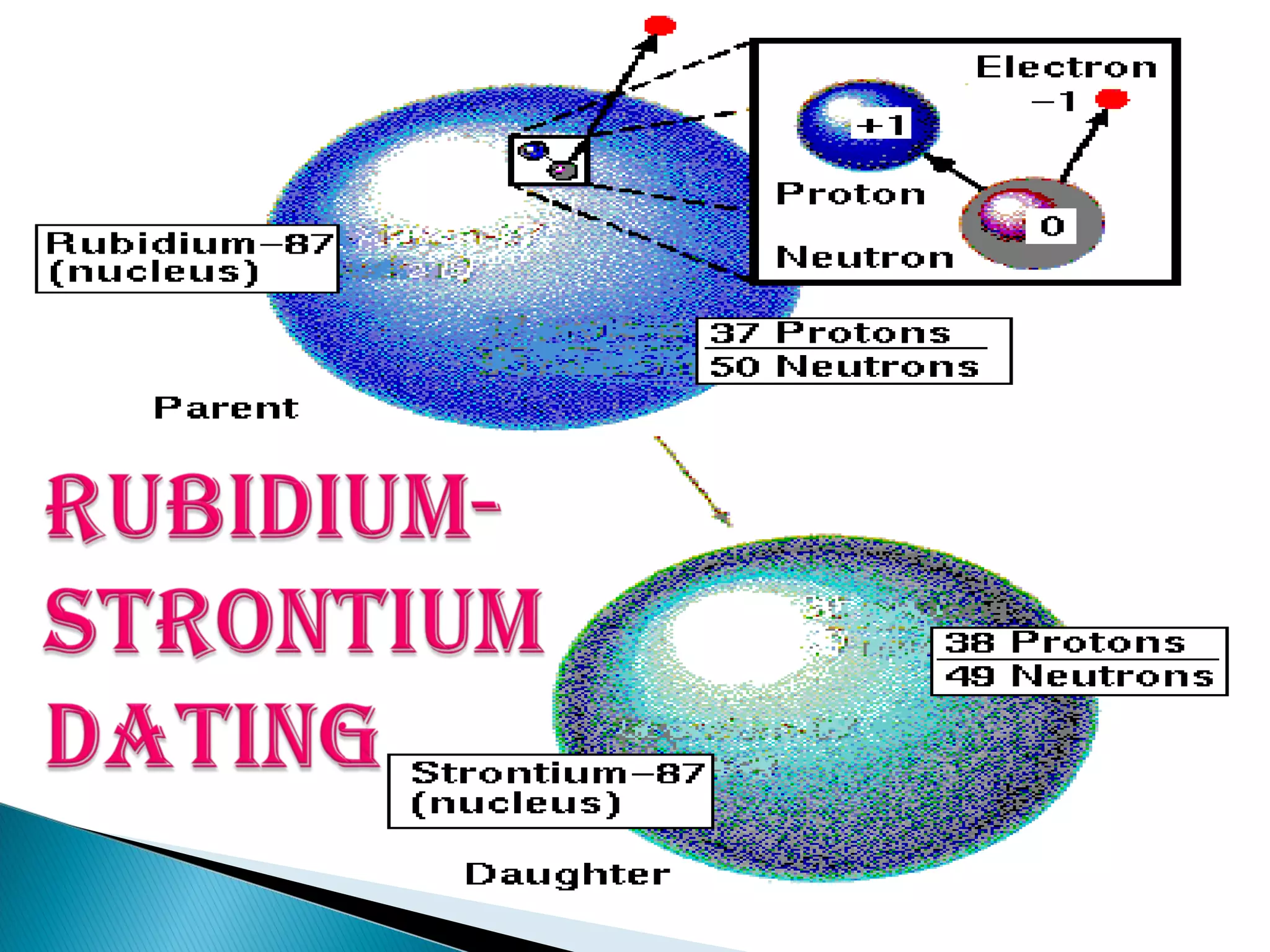
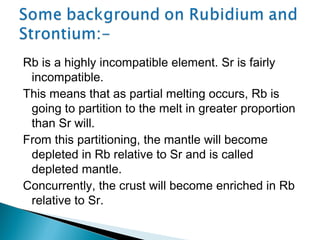
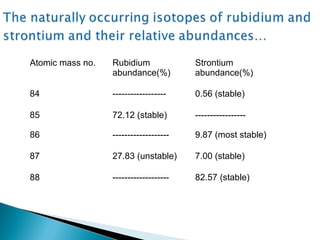
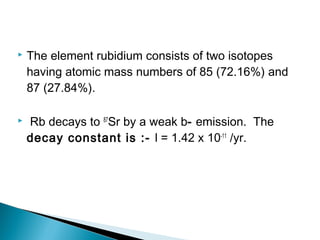
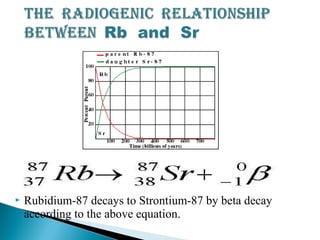
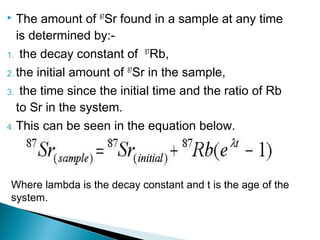
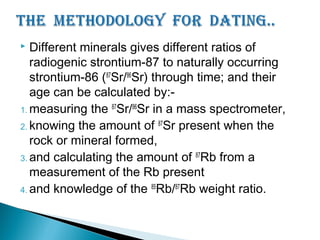
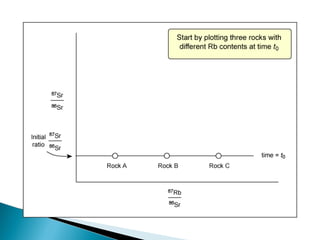
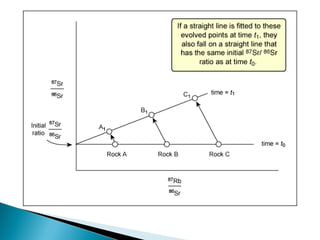
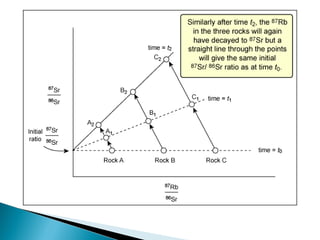
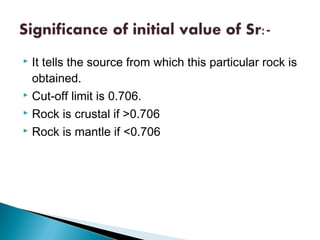
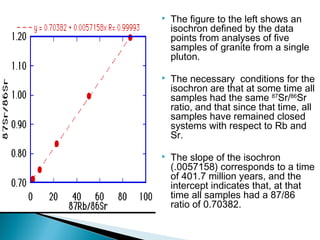
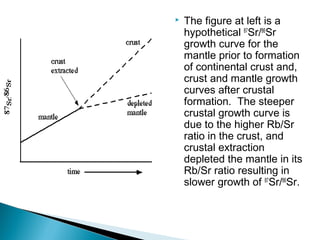
The Rb-Sr dating method uses the radioactive decay of rubidium-87 to strontium-87 to date rocks and minerals. It works by measuring the ratio of rubidium to strontium, and the ratio of radiogenic strontium-87 to strontium-86, in samples. By plotting these ratios on a graph called an isochron, scientists can determine the age of the sample from the slope of the line. The method relies on the samples forming a closed system and not being altered after formation. It is useful for determining the age of igneous and metamorphic rocks.