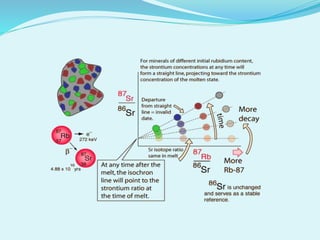
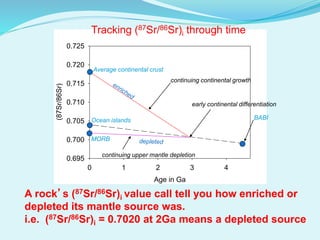
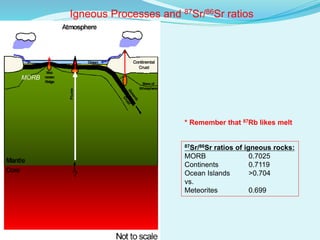
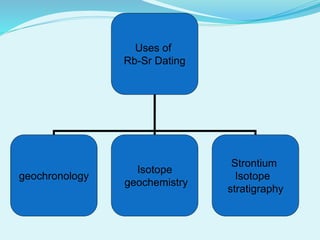
This document discusses rubidium-strontium dating, a radiometric dating technique that determines the age of rocks based on the radioactive decay of rubidium-87 to strontium-87. It describes the chemical properties of rubidium and strontium, how their relative abundances can vary in different rock types, and how the decay of rubidium-87 to strontium-87 can be used to calculate the age of a rock sample. It also discusses sources of error and applications of rubidium-strontium dating.



![87Rb-87Sr isochrons
87 87 87
86 86 86
( 1)
t
i
Sr Sr Rb
e
Sr Sr Sr
measured measured
when you crystallize a rock,
you will always have some Sr
present
Sample with
lower [Rb]
A schematic Rb-Sr isochron
Sample with
higher [Rb]
If x=(87Rb/86Sr)m
And y=(87Sr/86Sr)m
We have y=b+mx
Where intercept b=(87Sr/86Sr)i
And slope m=( )
b
- 1](https://image.slidesharecdn.com/jaifinal-141117134821-conversion-gate02/85/Rubidium-Strontium-Dating-8-320.jpg)