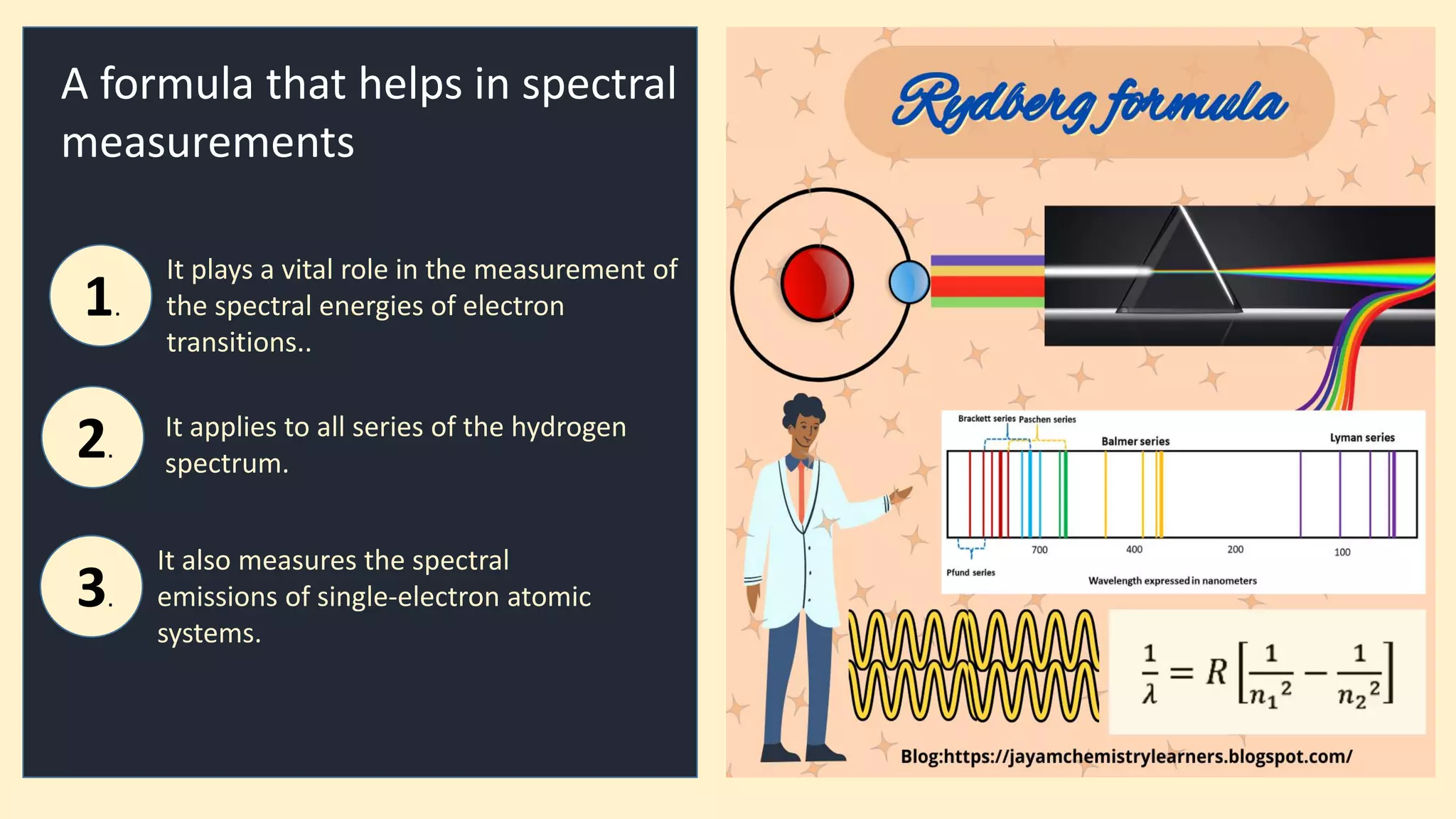
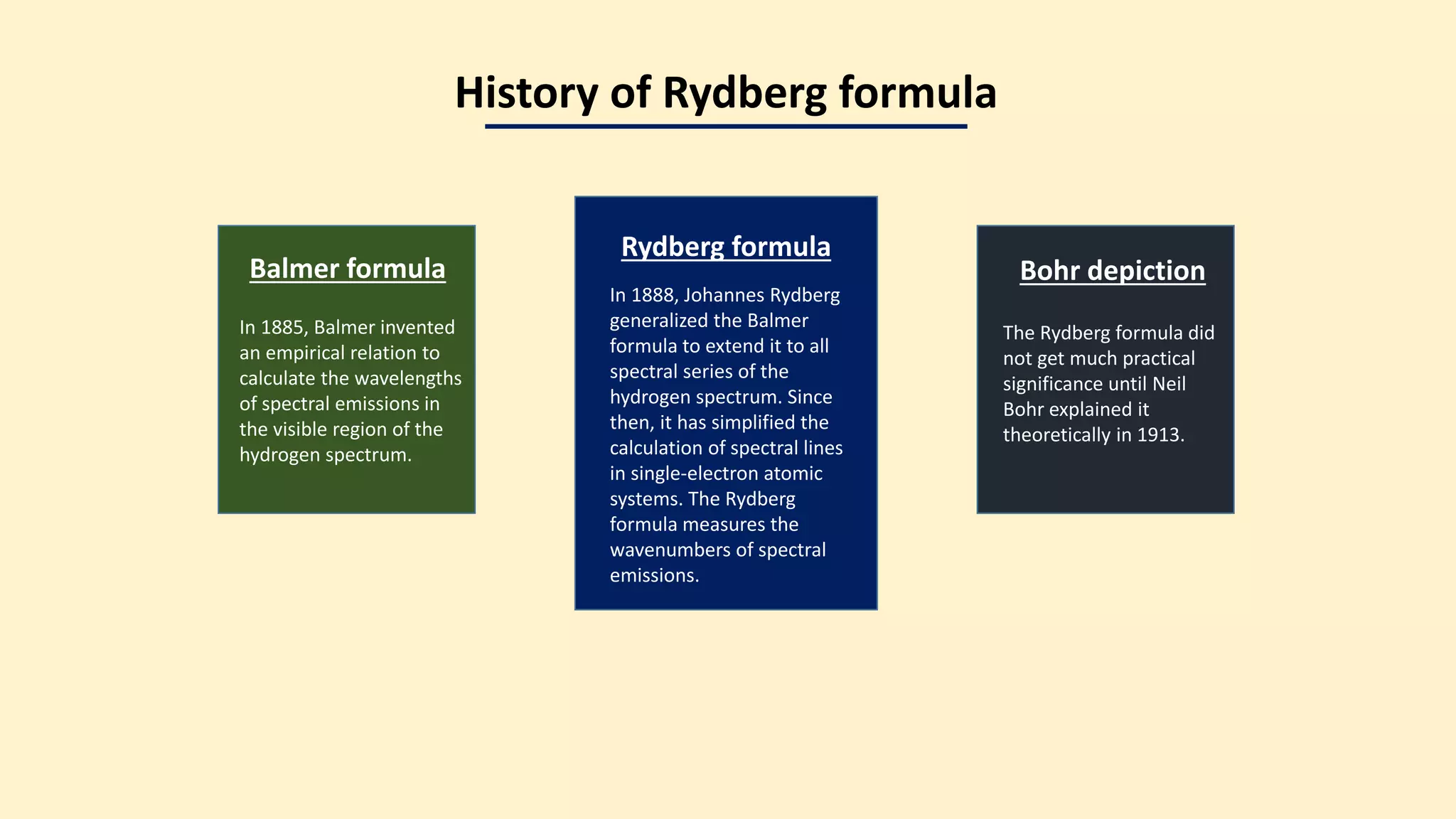
The document discusses the Rydberg equation, a key formula in atomic spectral analysis used to measure spectral energies from electron transitions, applicable to the hydrogen spectrum and single-electron atomic systems. It details the evolution of the Rydberg formula from Balmer's initial work and highlights its significance in estimating wavenumbers and understanding spectral emissions. The document also directs readers to further resources on chemistry topics and encourages engagement through social media.