Embed presentation
Download to read offline
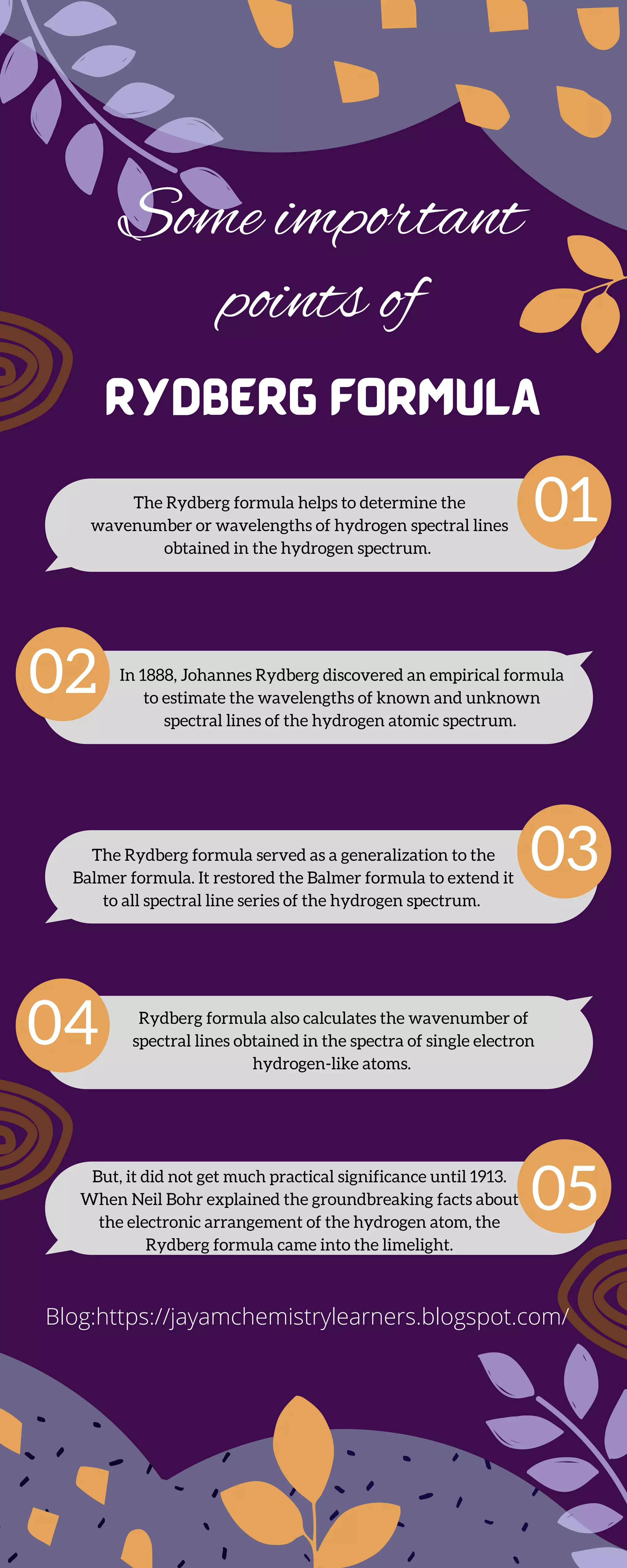
The Rydberg formula, discovered by Johannes Rydberg in 1888, estimates the wavelengths of hydrogen's spectral lines, extending the Balmer formula to all series in the hydrogen spectrum. Its practical significance increased in 1913 when Neil Bohr provided groundbreaking insights into the electronic structure of the hydrogen atom. Additionally, the formula can calculate the wavenumbers of spectral lines for single-electron hydrogen-like atoms.
