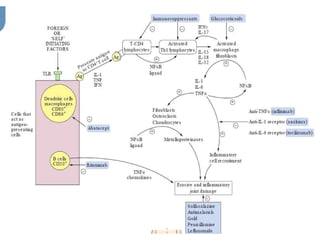
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by polyarthritis, primarily affecting joints like the wrists and fingers, driven by immune-related cells and cytokines. Treatment includes disease-modifying antirheumatic drugs (DMARDs), both non-biological and biological, such as methotrexate and TNF-α inhibitors, which suppress inflammation but can have significant adverse effects. Corticosteroids and NSAIDs are commonly used for symptom relief but do not alter the disease course.