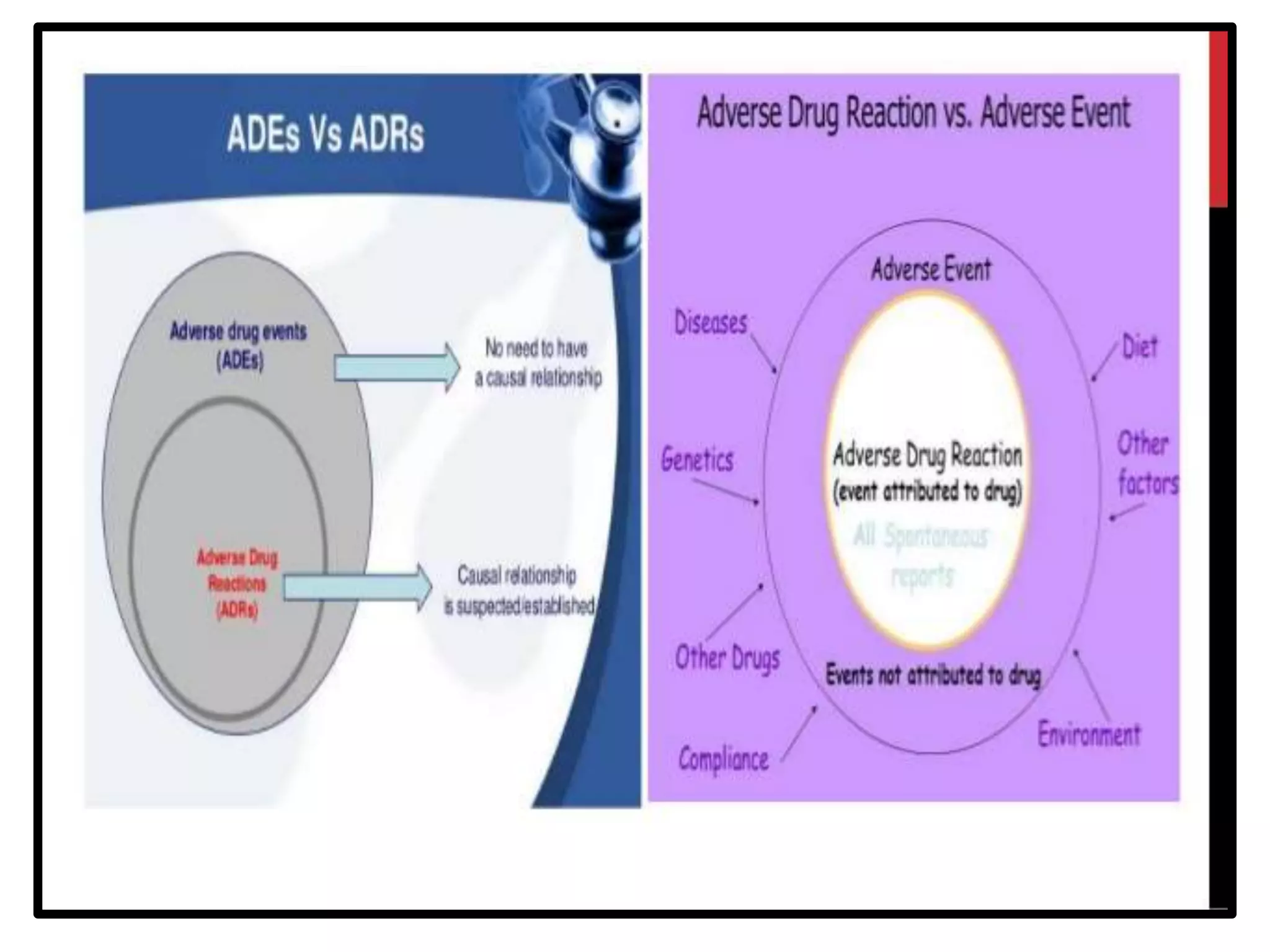
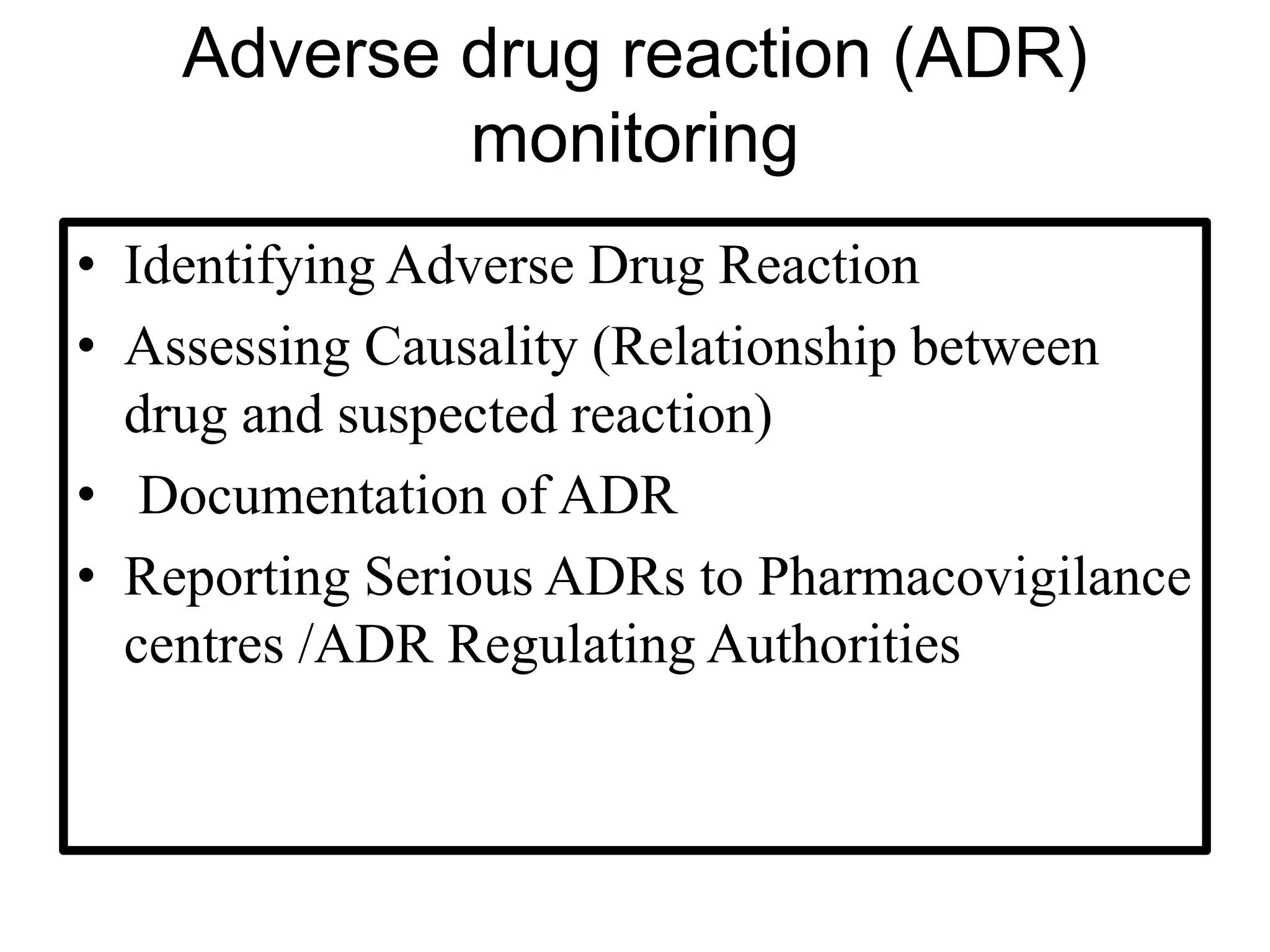
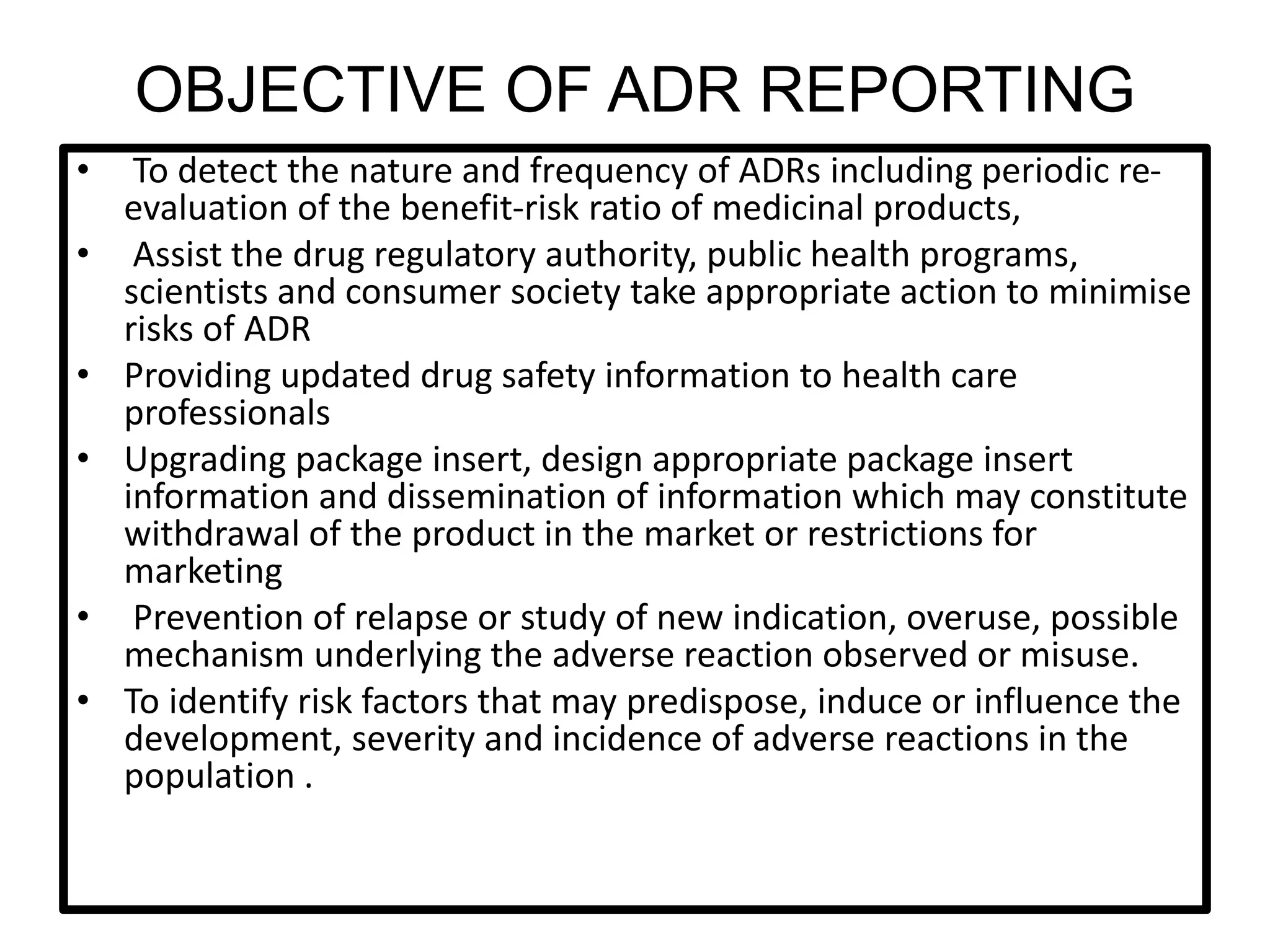
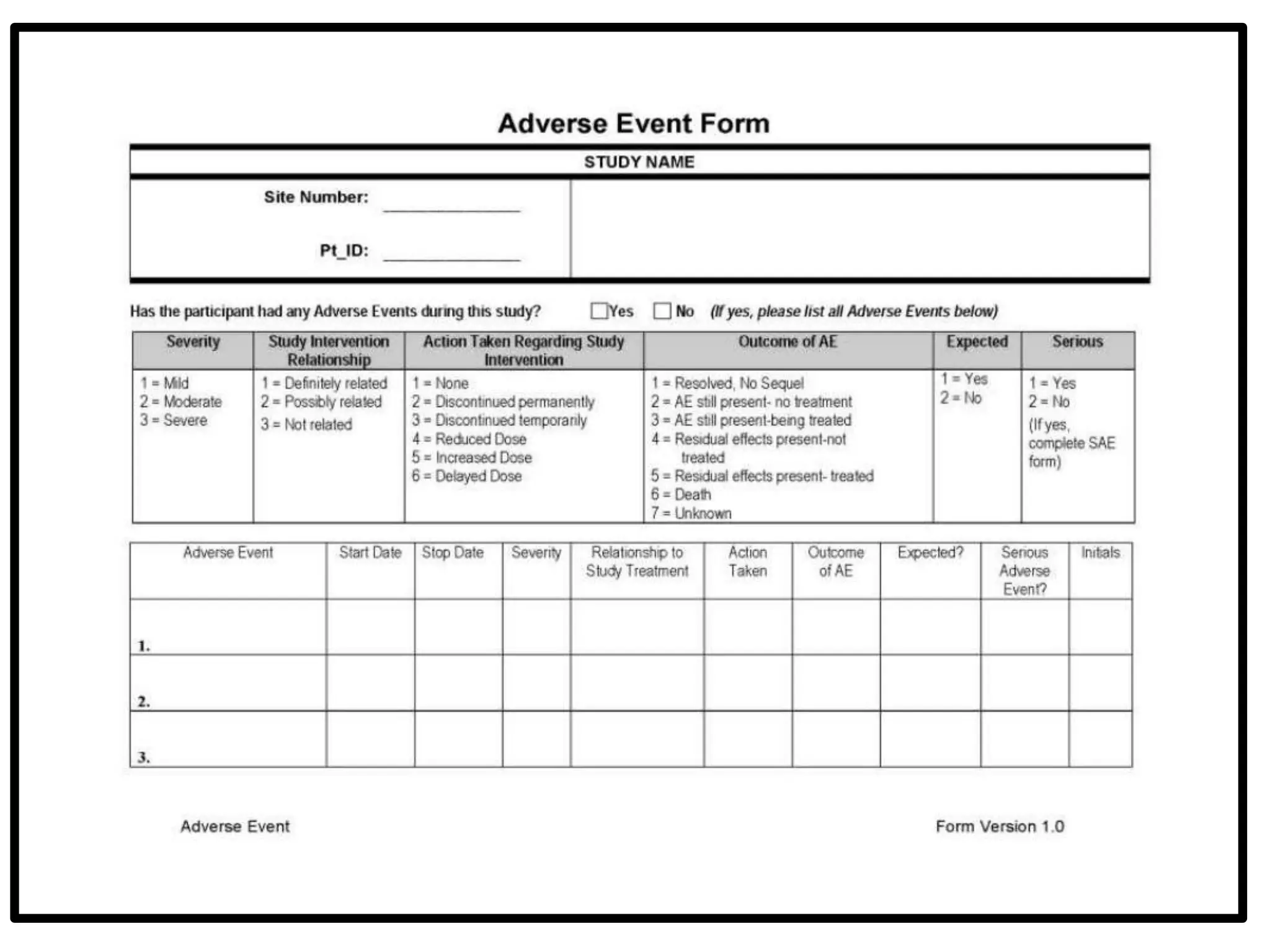
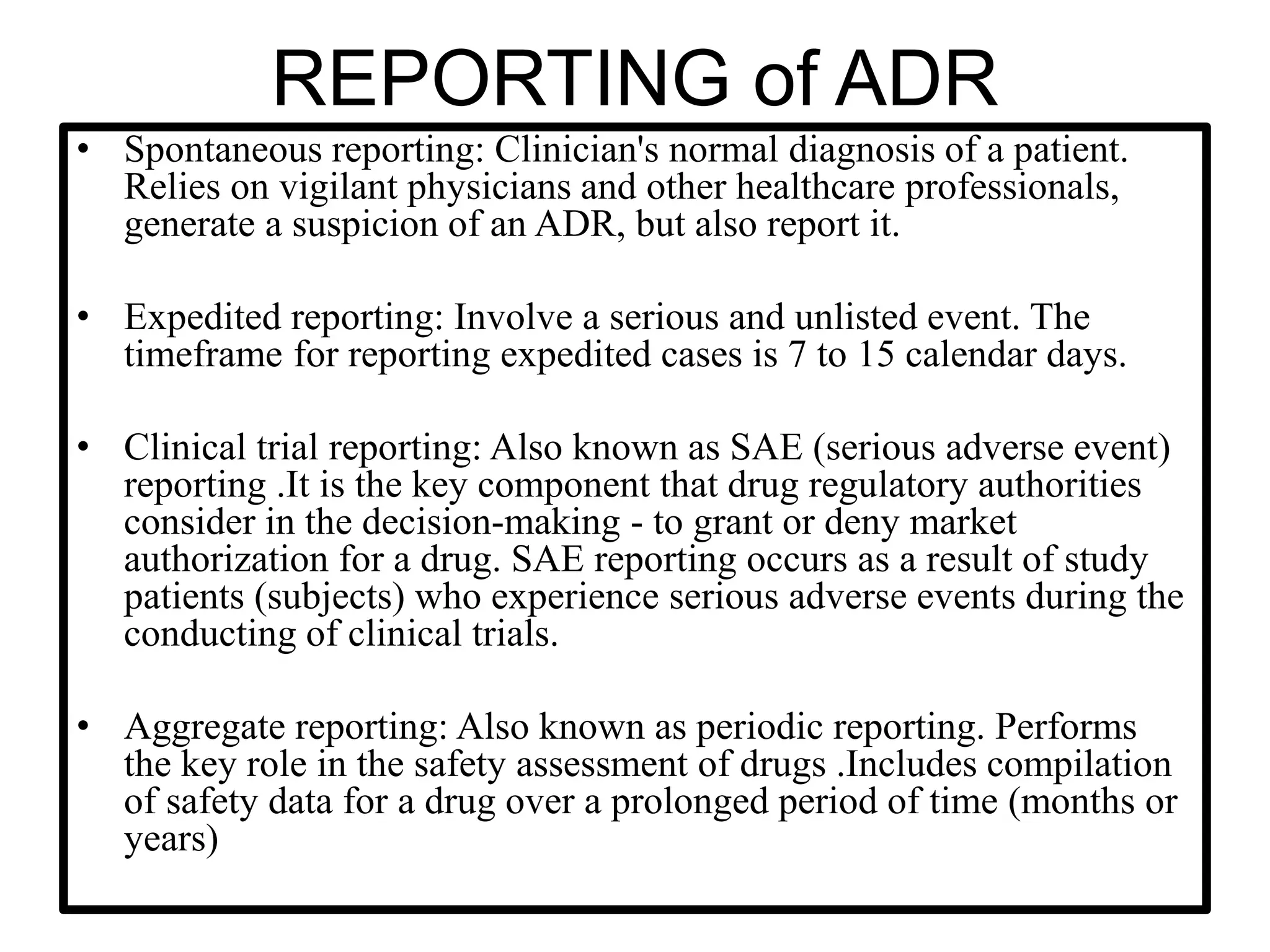
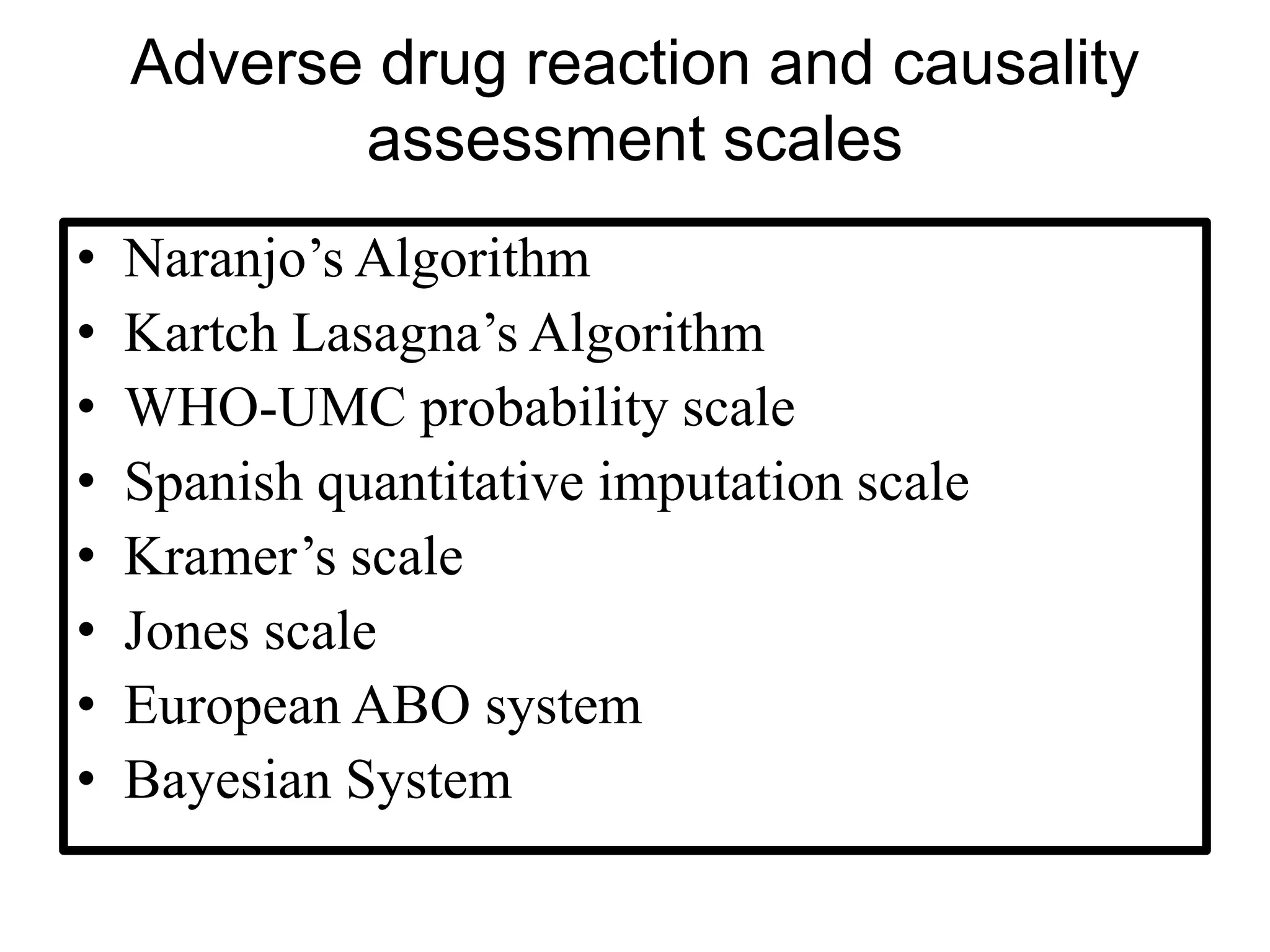
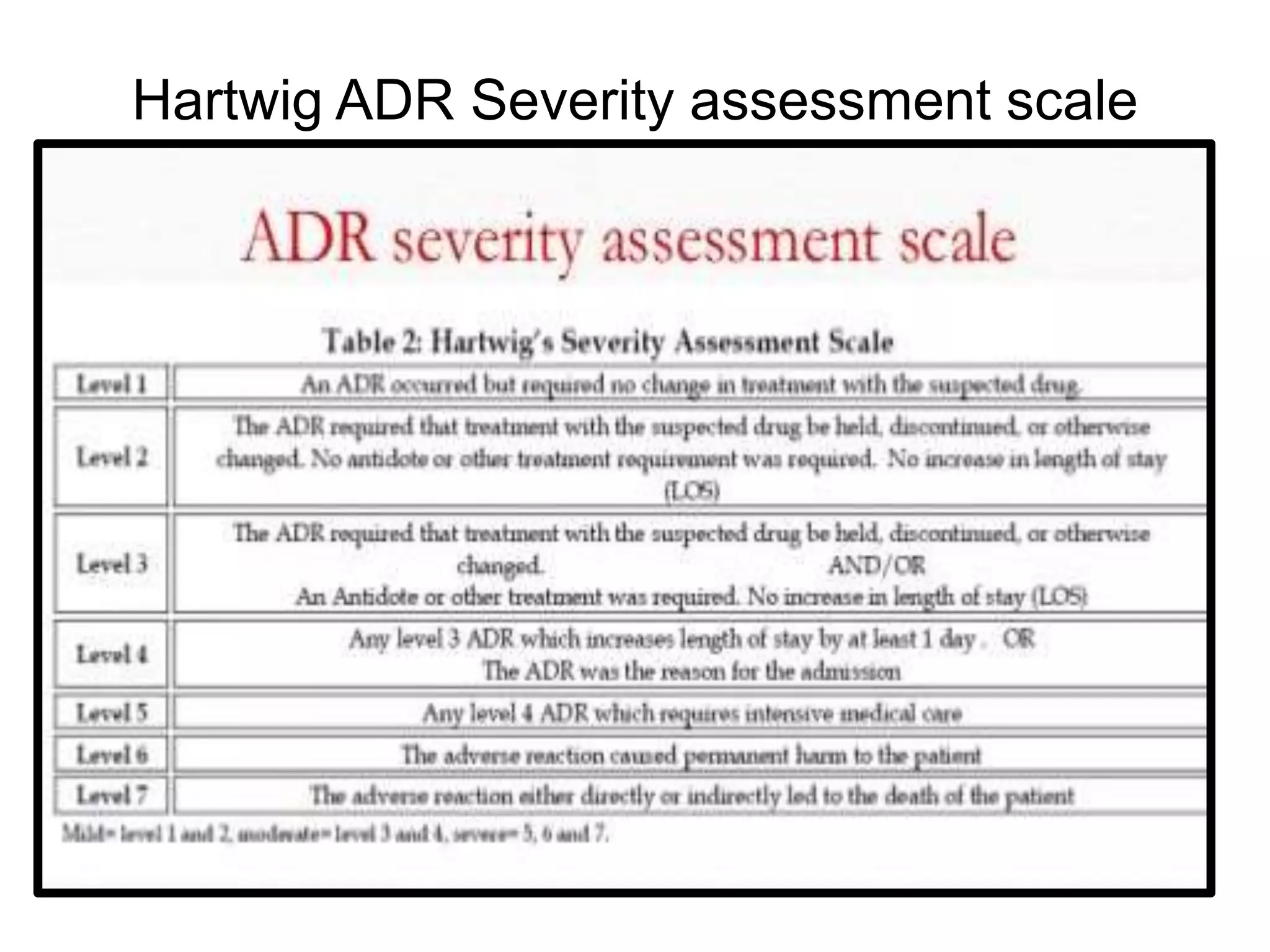
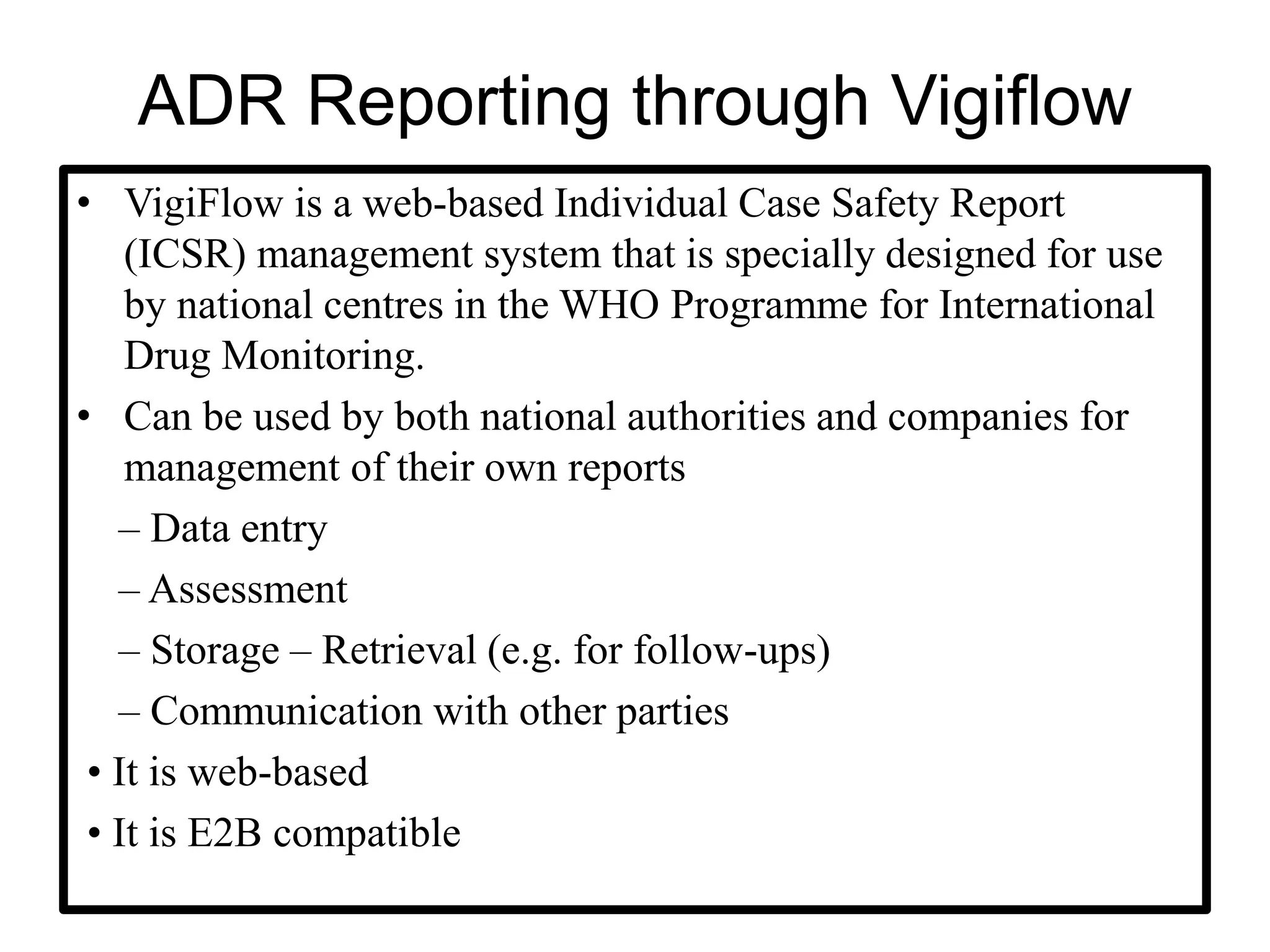
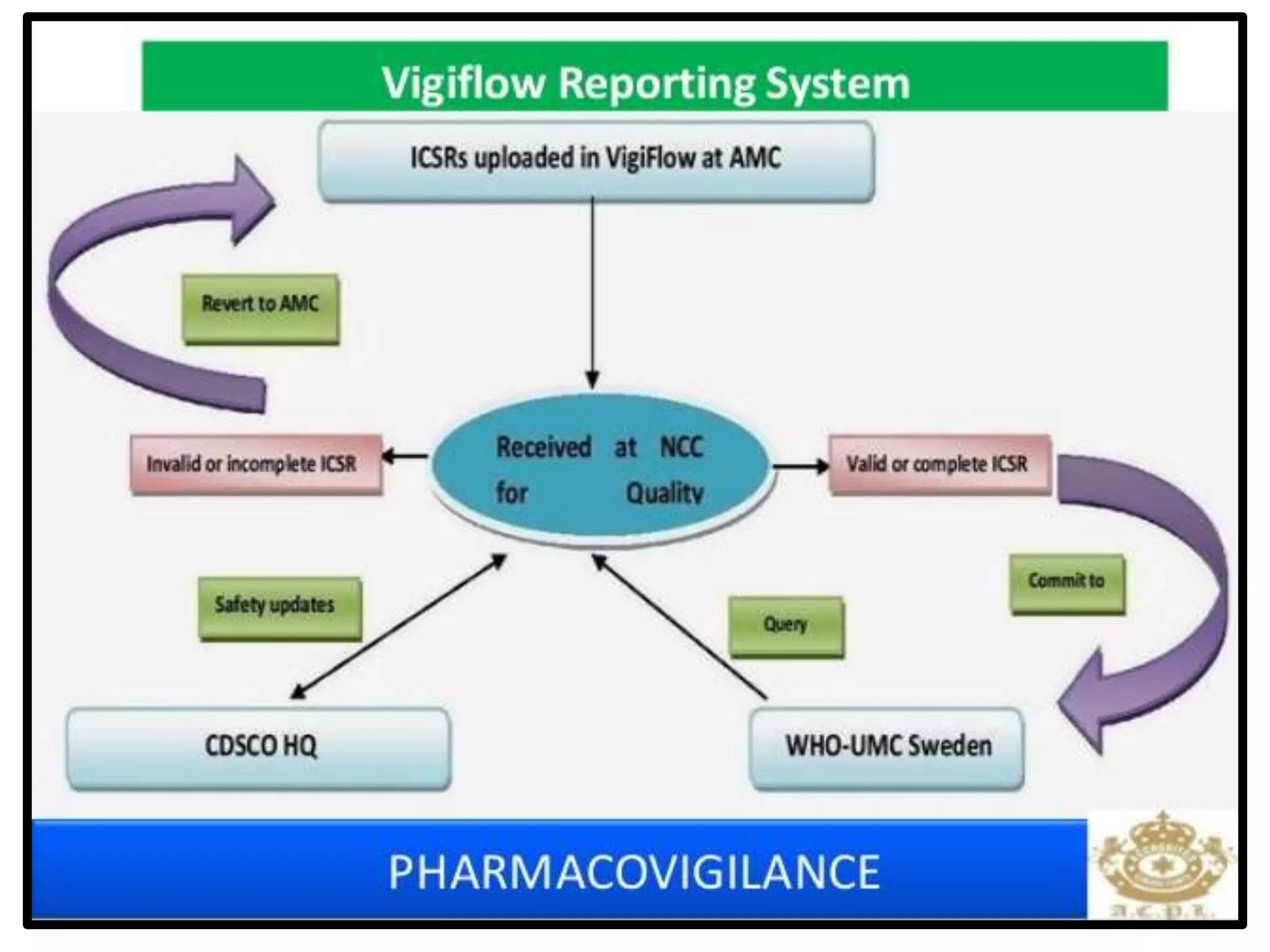
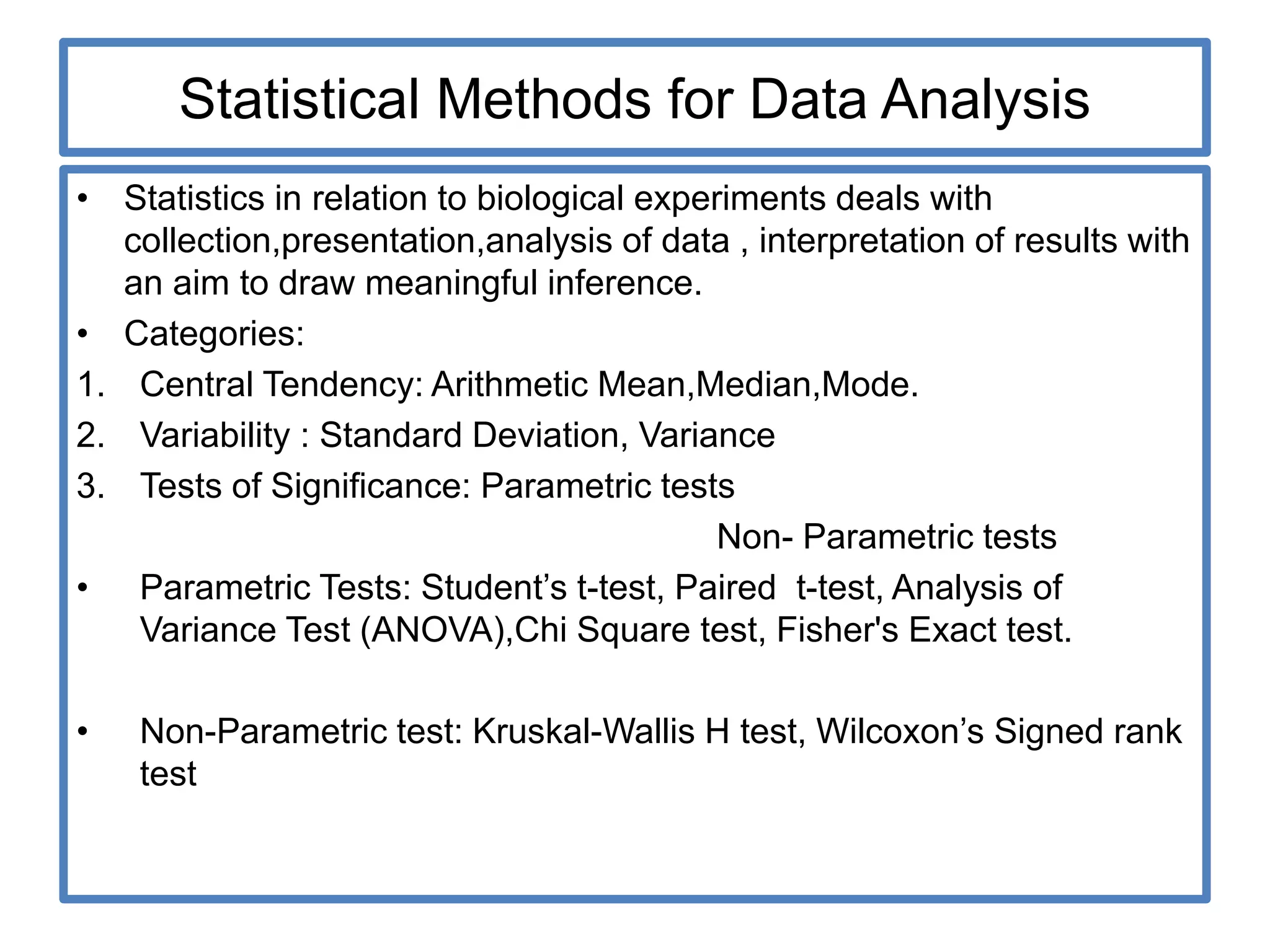
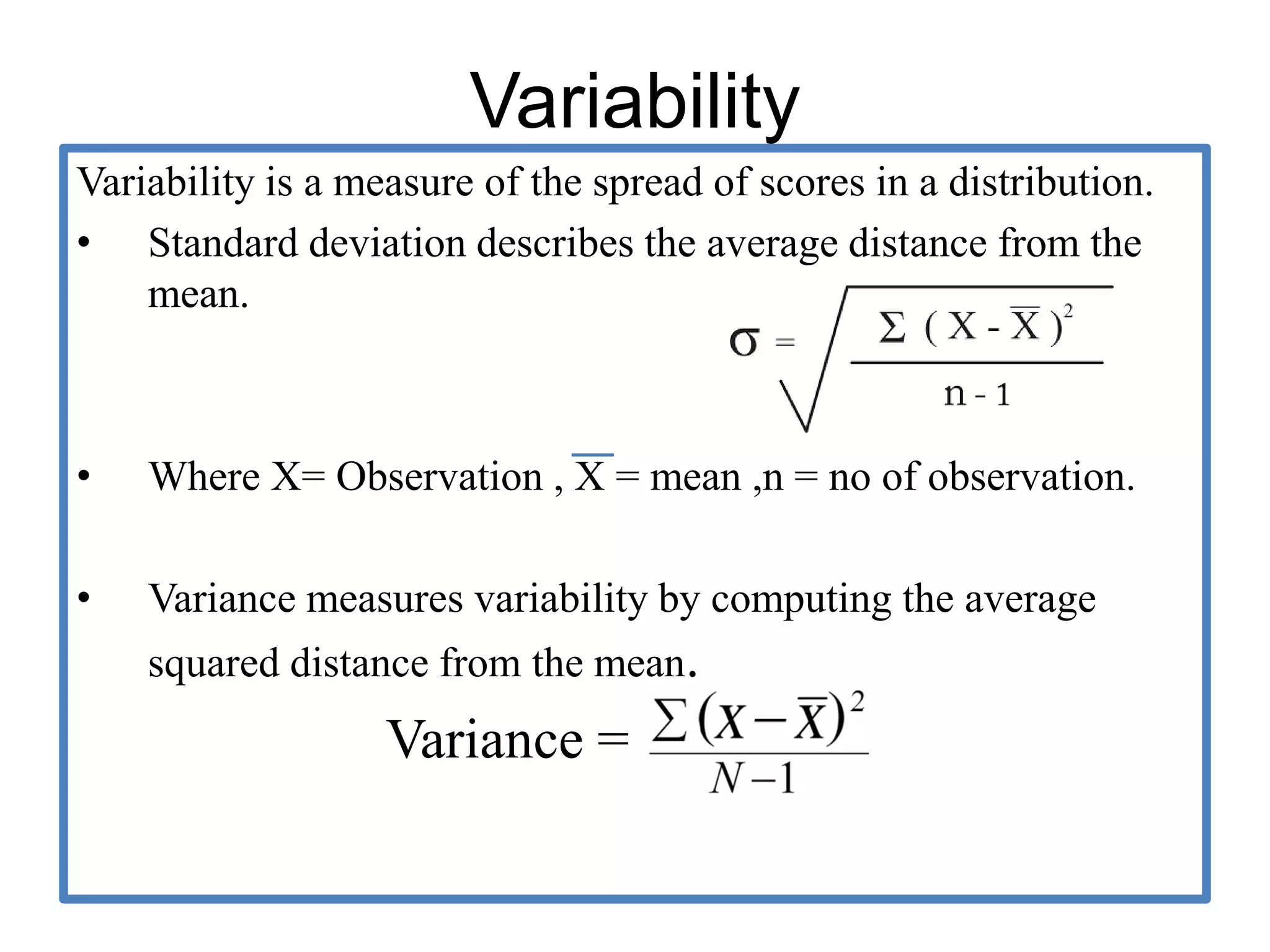
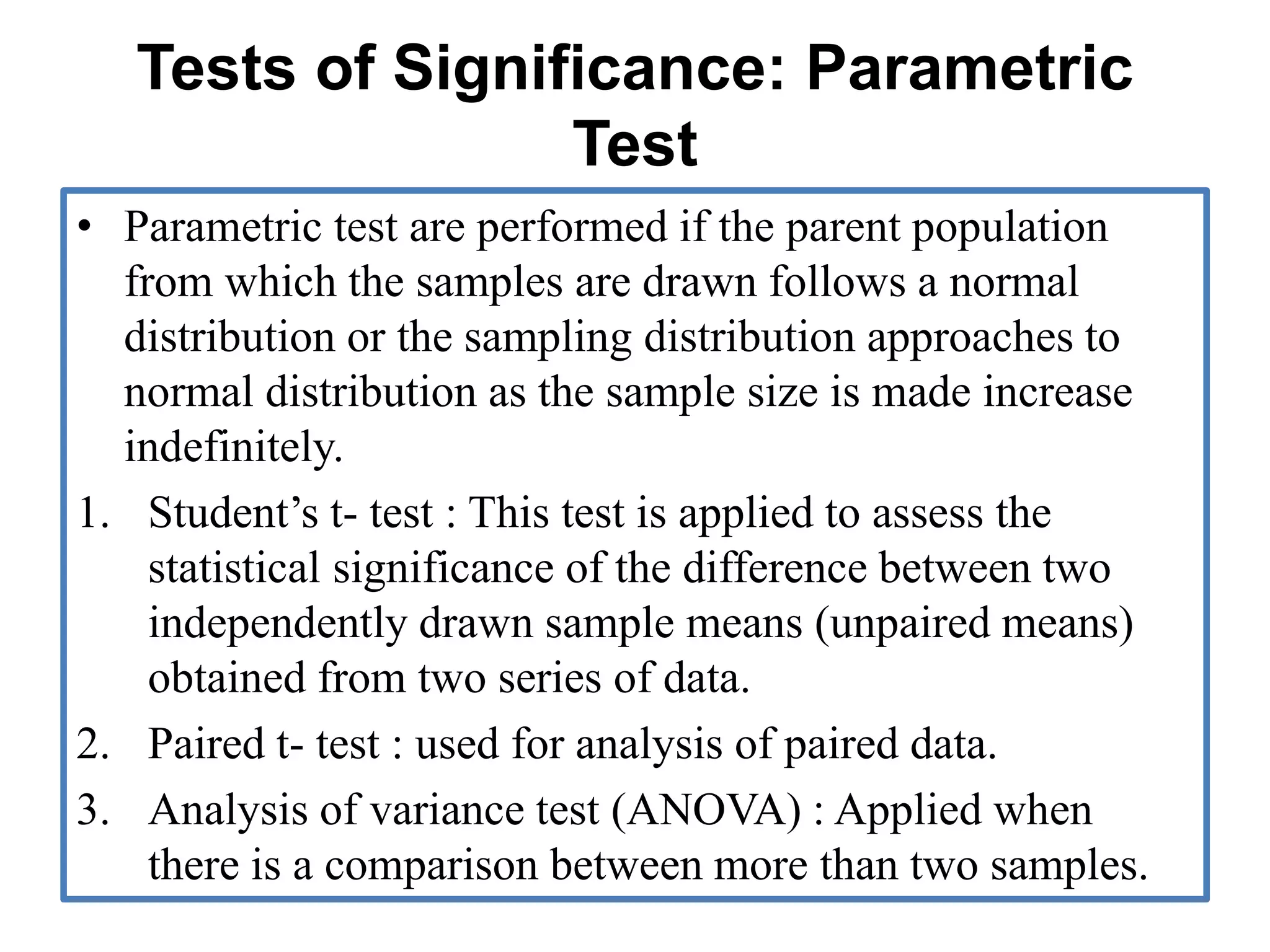
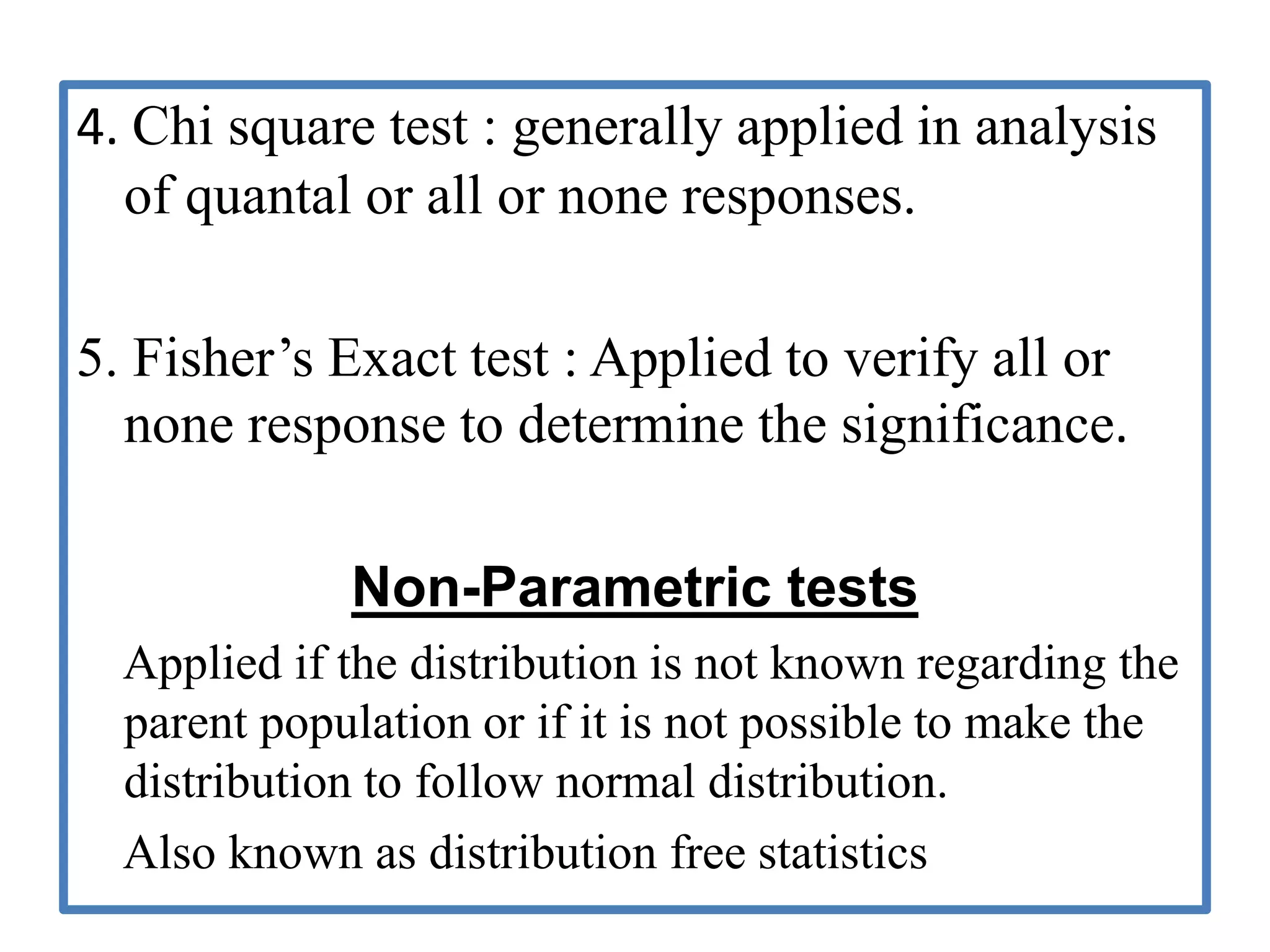
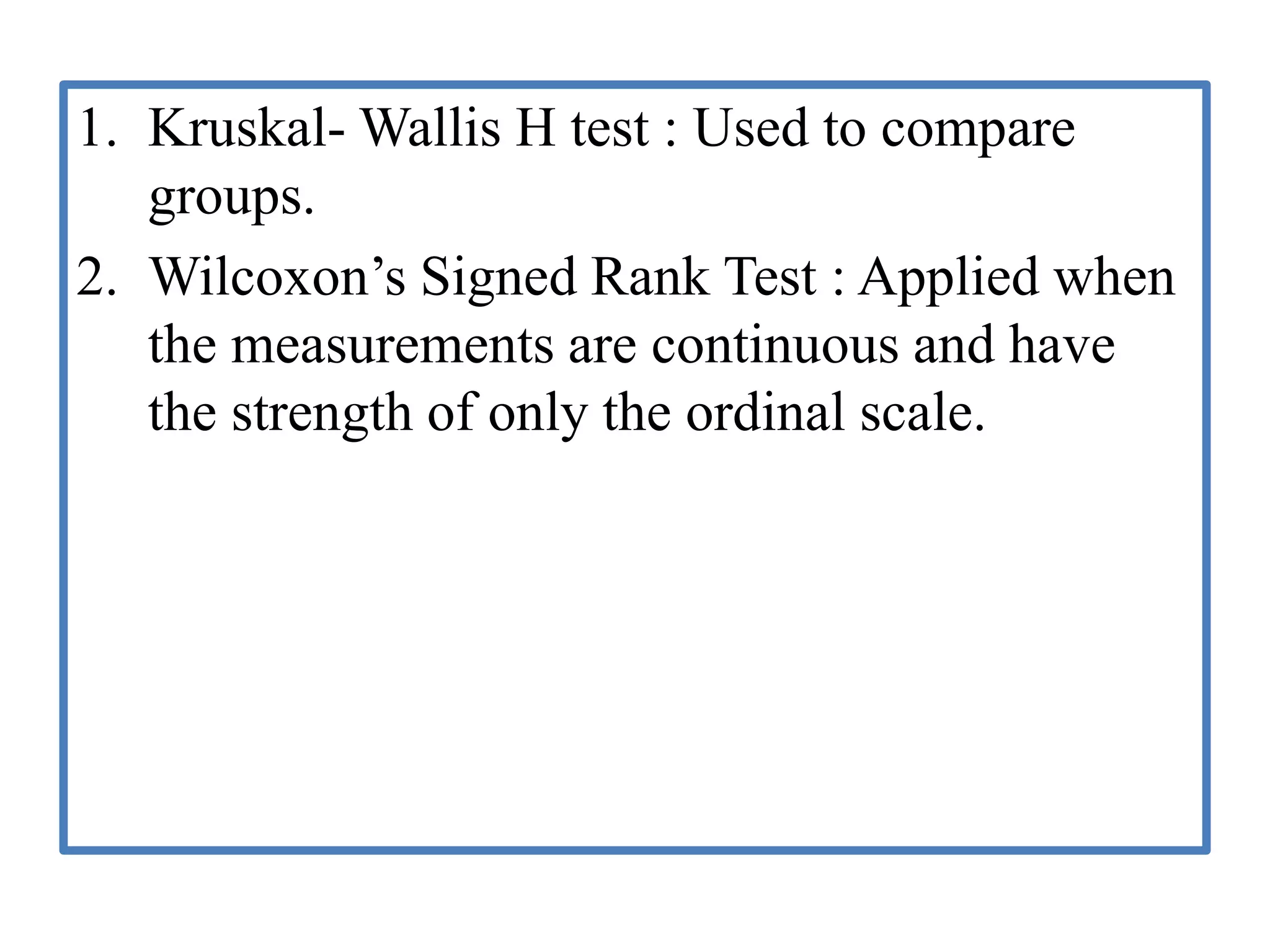
The document provides guidelines for adverse drug reaction (ADR) reporting and statistical methods used for safety data analysis. It defines ADRs and different types of ADR reporting such as spontaneous reporting, expedited reporting, and aggregate reporting. It also describes various pharmacovigilance methods used for ADR reporting including passive and active surveillance. Statistical analysis methods covered include measures of central tendency, variability, and parametric and non-parametric tests. Common scales for assessing ADR causality and severity are also mentioned.