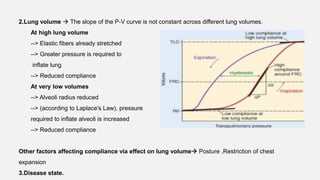
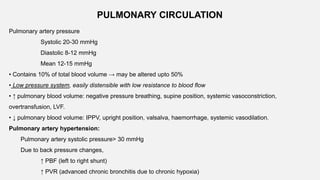
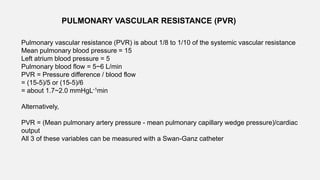
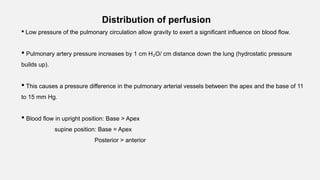
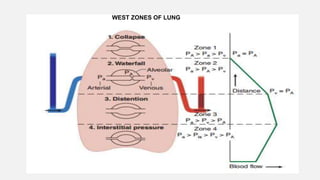
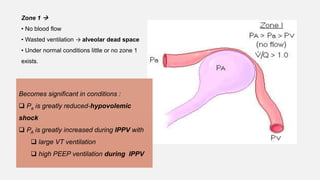
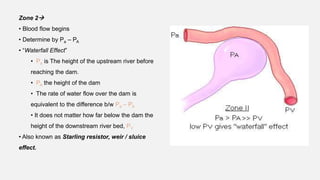
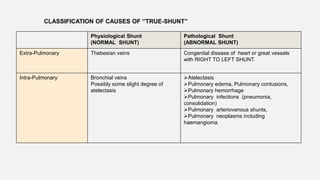
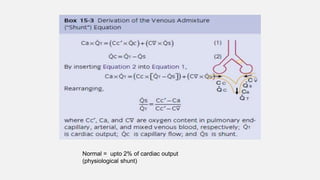
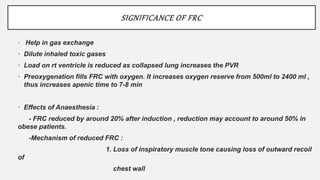
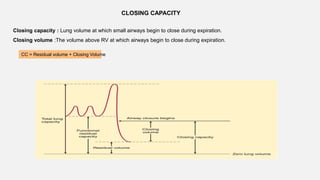
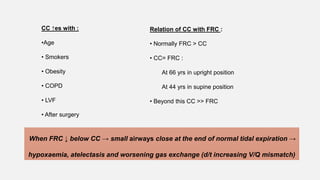
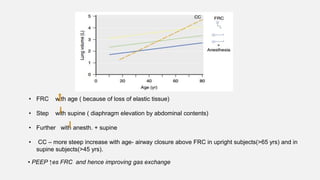
1. The document discusses the anatomy and physiology of the respiratory system including the structure of the lungs and airways, lung volumes and capacities, ventilation-perfusion ratios, and the control of breathing.
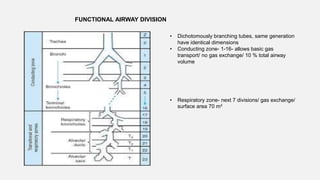
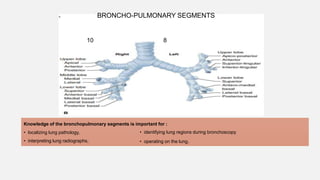
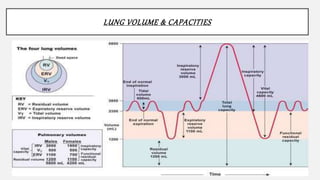
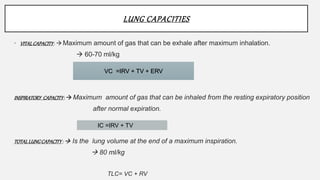
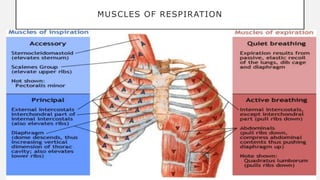
2. Key points covered are the tracheobronchial tree structure, functional airway division into conducting and respiratory zones, bronchopulmonary segments, factors affecting lung volumes such as tidal volume and vital capacity.
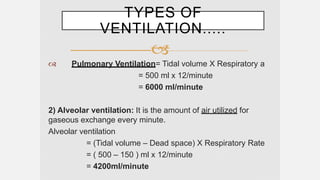
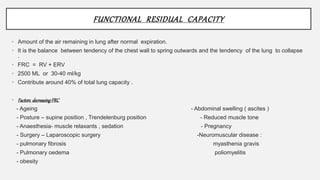
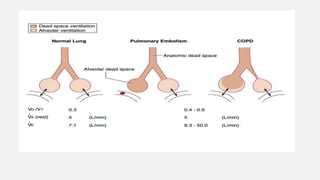
3. Concepts of dead space, alveolar ventilation, and the factors controlling respiration including the respiratory centers in the brainstem and response to changes in carbon dioxide and oxygen levels are summarized.











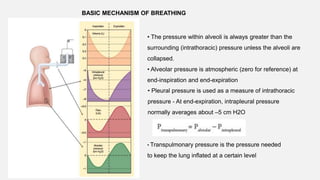
![Measurement of anatomical dead space
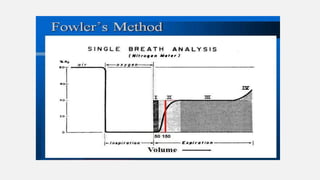
Fowler's method
Based on rapid dilution of gas already in lung (N2 or CO2) by inspired gas
(100% O2).
1.Single breath of 100% O2
2.During the following expiration, [N2] increases from 0% (pure dead space
gas) to equilibrium (pure alveolar gas) (i.e. plateau)
=> as per [N2] vs time graph
3.Using [N2] vs expired volume graph, anatomical dead space is taken to be
at the mid-point of the transition from conducting zone to gas exchange zone.](https://image.slidesharecdn.com/respiphysio1-230507084328-f0c2122f/85/RESPI-PHYSIO-1-pptx-22-320.jpg)


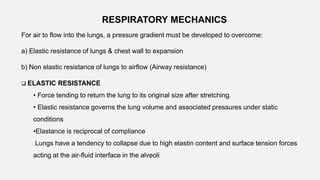
![Estimation of physiological dead space during anaesthesia with
passive ventilation : Cooper’s formula
V D/ VT = [33 + Age/3 ] %
Where PACO2 is alveolar CO2 tension
PECO2 is mixed expired CO2 tension
Bohr equation : Measures physiological dead space](https://image.slidesharecdn.com/respiphysio1-230507084328-f0c2122f/85/RESPI-PHYSIO-1-pptx-27-320.jpg)

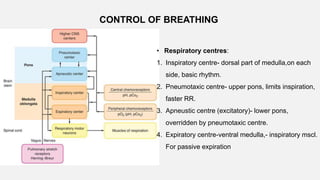
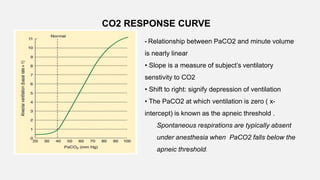

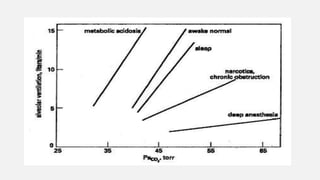
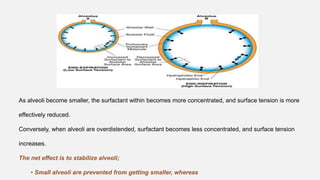
![• Central Chemoreceptors
• Lie on the anterolateral surface of the medulla
• Respond primarily to changes in cerebrospinal fluid (CSF) [H+]
• Blood–brain barrier is permeable to dissolved CO2 but not to bicarbonate ions.
• Acute changes in PaCO2 , but not in arterial [HCO3-], are reflected in CSF
• ↑PaCO2 → ↑ CSF [H+] concentration → activate the chemoreceptors → stimulates
adjacent respiratory medullary centers → alveolar ventilation ↑→ PaCO2 ↓ back to
normal.](https://image.slidesharecdn.com/respiphysio1-230507084328-f0c2122f/85/RESPI-PHYSIO-1-pptx-34-320.jpg)