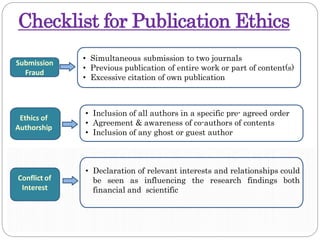
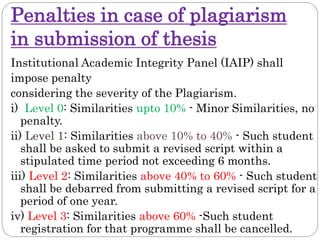
This document discusses developing research protocols. It defines research and clinical research protocols. Protocols are required to clarify the research question, compile existing knowledge, formulate hypotheses and objectives, decide on study design, and clarify ethical considerations. Key parts of a protocol include the study title, introduction, methodology, time frames and budget, and references. The document emphasizes following ethical guidelines to protect participants. It provides a checklist for publication ethics addressing approval, plagiarism, data accuracy, submission practices, authorship, and conflicts of interest. Penalties for plagiarism in theses are also outlined.







![Indian Council of Medical Research
introduced Ethical Guidelines for
Research on Human Participants
NATIONAL GUIDELINES
2000, ICMR
guidelines
All institutions in the country which carry
out any form of biomedical research
involving human beings should follow these
guidelines in letter and spirit to protect
safety and well being of all individuals.
2006, Revised
ICMR
guidelines
There are
several other
national
guidelines
available
Genome Policy and Genetic Research
[2000], Indian GCP [2001], Amendment of
Drugs and Cosmetics Act [2002], Assisted
Reproductive Technology [2005], Stem Cell
Research and Bio-banking [2006]](https://image.slidesharecdn.com/researchprotocolbysandeep-230104183751-c789d797/85/research-protocol-By-Sandeep-pptx-8-320.jpg)