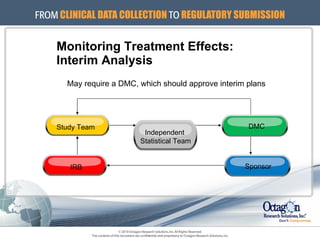
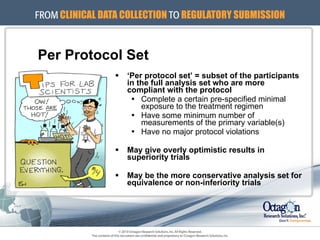
The document outlines the ICH E9 guideline on statistical principles for clinical trials, emphasizing the importance of proper statistical practices in trial design, conduct, analysis, and evaluation. It addresses minimizing bias, maximizing precision, and ensuring robust protocols while distinguishing between exploratory and confirmatory trials. The guideline serves as a roadmap for statisticians and researchers to apply appropriate statistical methods to ensure the validity of trial outcomes.