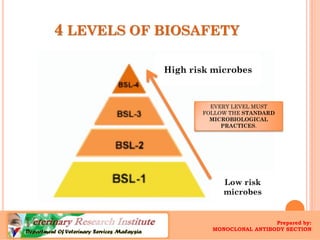
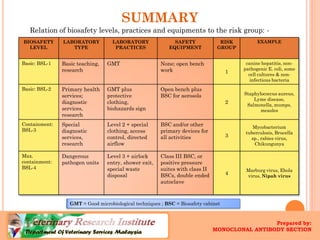
The document defines biosafety levels and containment controls for working with biological agents. There are four biosafety levels that provide increasing containment based on the risk group of the agent. BSL-1 is for low risk agents and allows open bench work, while BSL-4 is for dangerous exotic agents and requires the highest level of containment like positive pressure suits. Each level has specific laboratory practices, safety equipment, and facility requirements to safely contain biological agents and protect laboratory workers from exposure.