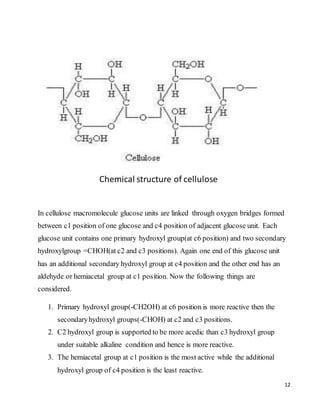
The document provides an overview of reactive dyes:
1) Reactive dyes chemically bond to fibers through reactive groups that form covalent bonds with hydroxyl or amino groups on fibers like cotton, polyamide, and wool.
2) They were first invented in 1956 and provided brighter colors and better fastness than previous dyes.
3) Reactive dyes are now widely used for cellulosic fibers due to their brighter colors, good fastness properties, and simpler dyeing process compared to other dyes.