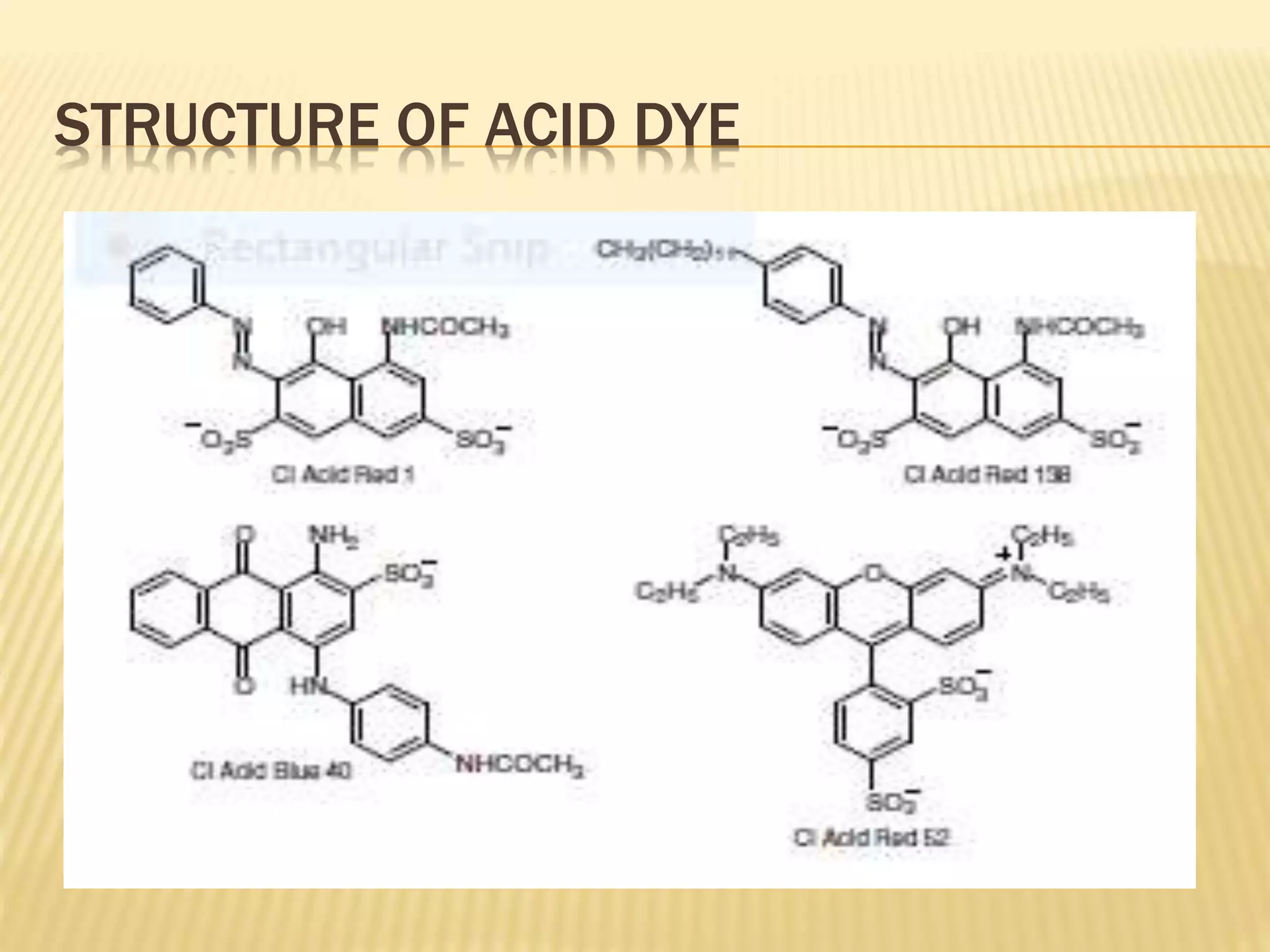
This document provides information about acid dyes. It begins with an introduction to acid dyes, noting that they are large dyes containing sulfonic or carboxylic acid groups that dye protein fibers like wool from acid solutions. It then discusses the properties of acid dyes, including that they are water soluble and have affinity for protein and nylon fibers. The document also covers the classification, structure, and dyeing processes for acid dyes. In particular, it differentiates between types of acid dyes like levelling, fast, milling, and super-milling dyes based on their molecular size and dyeing characteristics.