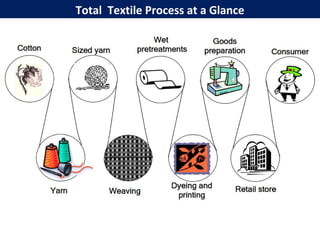
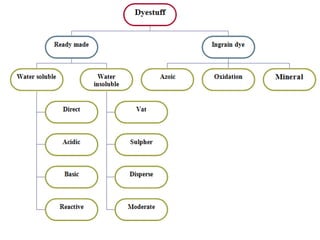
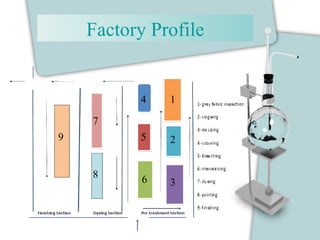
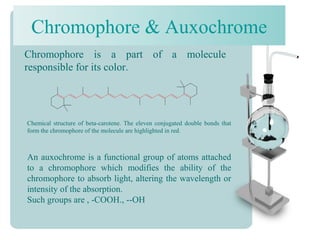
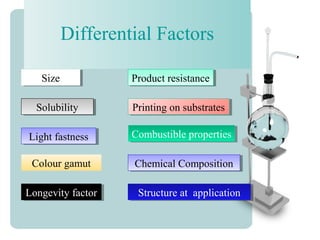
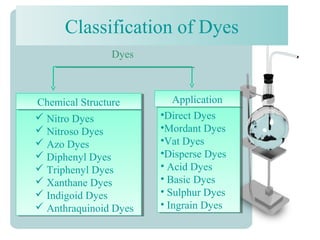
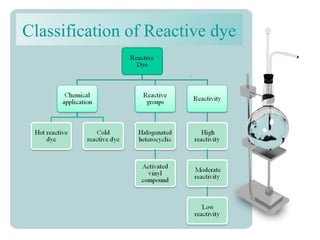
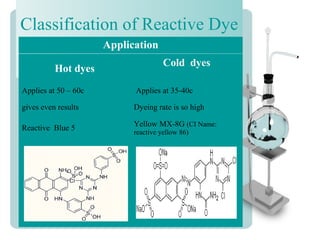
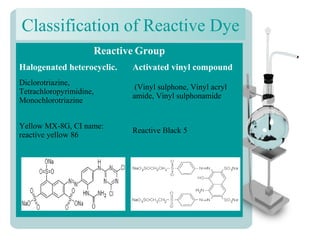
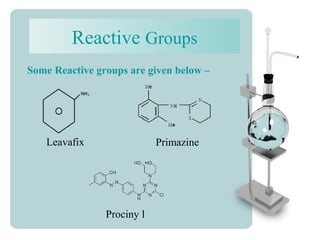
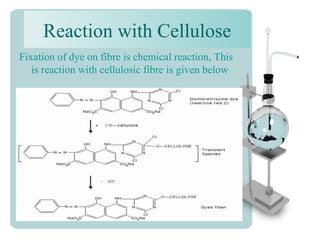
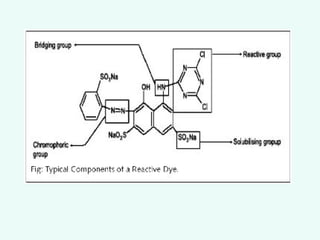
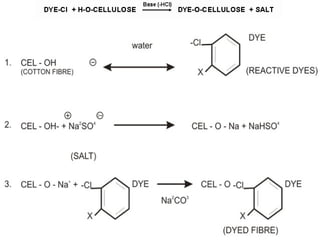
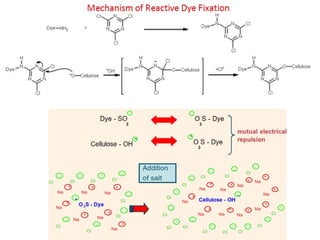
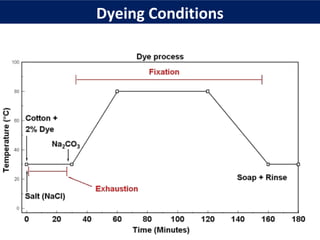
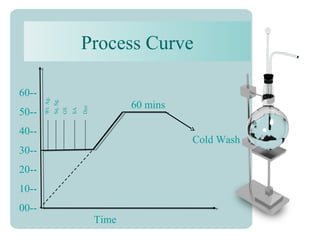
This document provides an overview of dyes and pigments, with a focus on reactive dyes. It defines dyes and pigments, describes their classification and color-producing factors. Reactive dyes are discussed in more detail, including their classification based on reactivity and reactive groups. The process of reactive dyeing is summarized, including dyeing conditions, the approach of reactive dyes to fibers, and their chemical reaction with cellulose fibers. A sample process curve is also shown. Finally, the document provides a profile of a wet processing plant to illustrate dyeing operations.