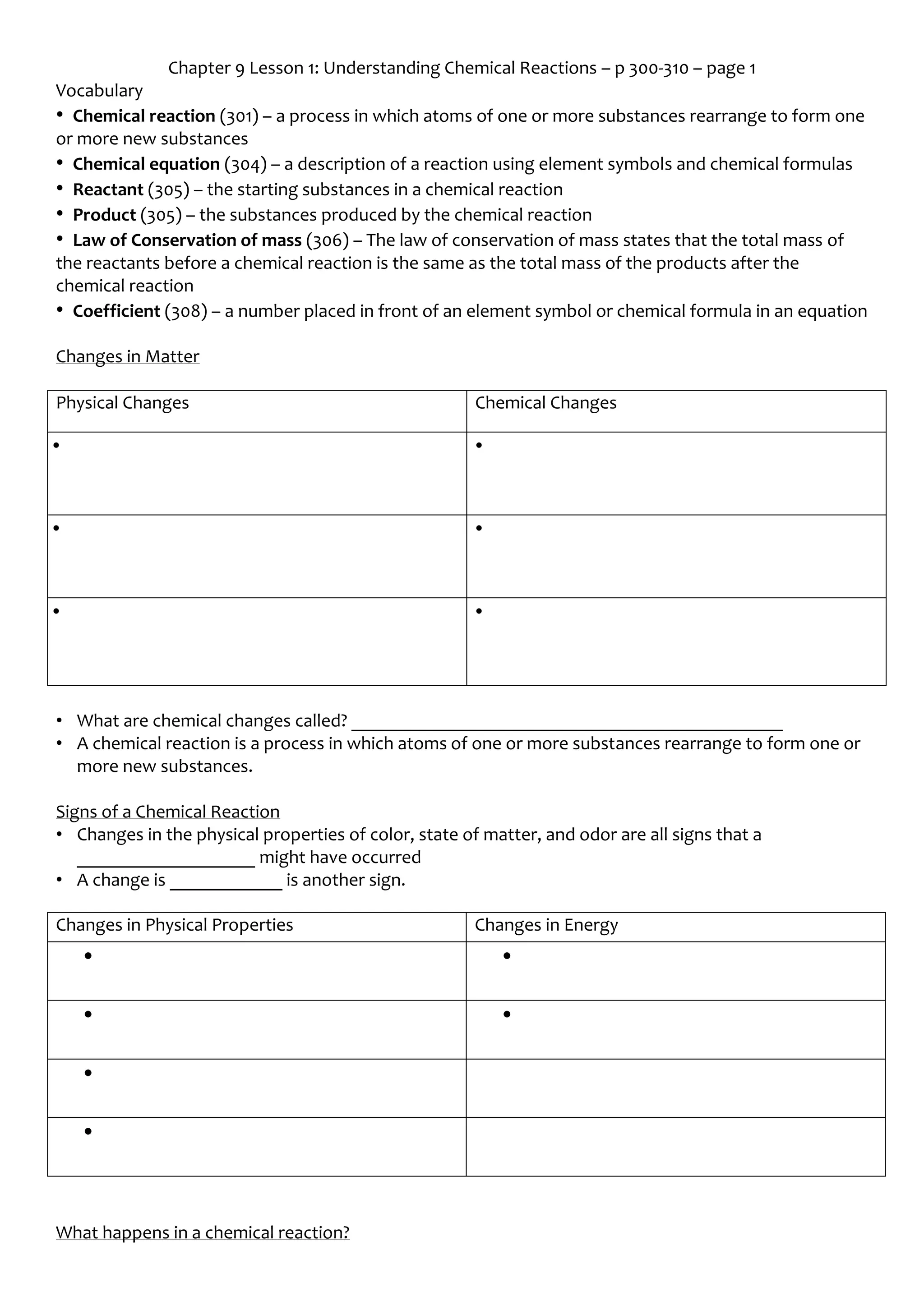
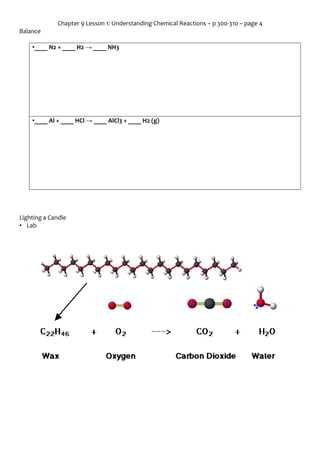
Chapter 9 Lesson 1 covers the fundamentals of chemical reactions, including key vocabulary such as reactants, products, and the law of conservation of mass. It explains the processes of how atoms rearrange to form new substances, the significance of chemical equations, and the importance of balancing these equations. The lesson highlights the signs of chemical reactions and the principles governing the conservation of mass during such transformations.