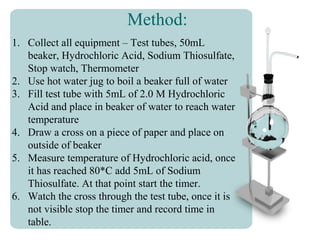
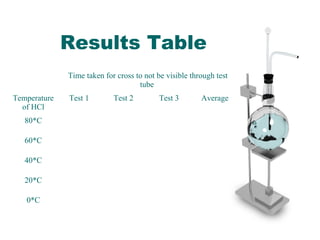
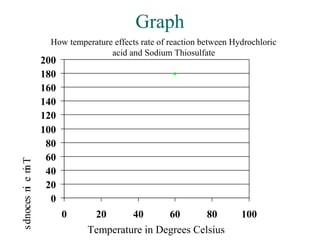
This document outlines an experiment to determine how temperature affects the rate of reaction between sodium thiosulfate and hydrochloric acid. It describes the independent variable as the temperature of the hydrochloric acid ranging from 0°C to 80°C. The dependent variable is the time for a cross drawn on paper to become invisible when placed behind a test tube containing the reaction. The method involves timing how long it takes for the cross to disappear at each temperature, with results recorded in a table. The conclusion states that higher temperatures led to faster reaction times, supporting the particle collision theory that increasing temperature causes particles to move faster and collide more frequently.